Ice
Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color.
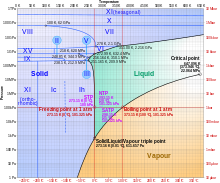
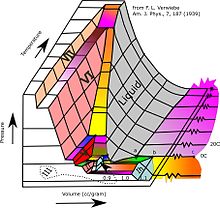
The most common phase transition to ice Ih occurs when liquid water is cooled below 0 °C (273.15 K, 32 °F) at standard atmospheric pressure.
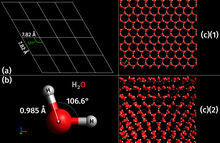
[6] An unusual property of water is that its solid form—ice frozen at atmospheric pressure—is approximately 8.3% less dense than its liquid form; this is equivalent to a volumetric expansion of 9%.
Instead, a sheltered environment for animal and plant life is formed beneath the floating ice, which protects the underside from short-term weather extremes such as wind chill.
[10] When sea water freezes, the ice is riddled with brine-filled channels which sustain sympagic organisms such as bacteria, algae, copepods and annelids.
[12][8] As with water, ice absorbs light at the red end of the spectrum preferentially as the result of an overtone of an oxygen–hydrogen (O–H) bond stretch.
Since absorption is cumulative, the color effect intensifies with increasing thickness or if internal reflections cause the light to take a longer path through the ice.
[21] With care, at least fifteen of these phases (one of the known exceptions being ice X) can be recovered at ambient pressure and low temperature in metastable form.
However, the significance of this hypothesis is disputed by experiments showing a high coefficient of friction for ice using atomic force microscopy.
[42][43] The World Meteorological Organization defines several kinds of ice depending on origin, size, shape, influence and so on.
As they become somewhat larger and more consistent in shape and cover, the water surface begins to look "oily" from above, so this stage is called grease ice.
They form within eddy currents, and their position results in asymmetric melting, which makes them continuously rotate at a low speed.
This kind of ice may contain large air pockets under a thin surface layer, which makes it particularly hazardous to walk across it.
In the United States, a quarter of winter weather events produce glaze ice, and utilities need to be prepared to minimize damages.
[76] Hail forms in storm clouds when supercooled water droplets freeze on contact with condensation nuclei, such as dust or dirt.
Ice pellets typically form alongside freezing rain, when a wet warm front ends up between colder and drier atmospheric layers.
[93] More generally, water vapor depositing onto surfaces due to high relative humidity and then freezing results in various forms of atmospheric icing, or frost.
[95] In Antarctica, the temperatures can be so low that electrostatic attraction is increased to the point hoarfrost on snow sticks together when blown by wind into tumbleweed-like balls known as yukimarimo.
[101][102] In recent decades, irrigation sprinklers have been calibrated to spray just enough water to preemptively create a layer of ice that would form slowly and so avoid a sudden temperature shock to the plant, and not be so thick as to cause damage with its weight.
Yakhchals often included a qanat and a system of windcatchers that could lower internal temperatures to frigid levels, even during the heat of the summer.
[106][107] There were thriving industries in 16th–17th century England whereby low-lying areas along the Thames Estuary were flooded during the winter, and ice harvested in carts and stored inter-seasonally in insulated wooden houses as a provision to an icehouse often located in large country houses, and widely used to keep fish fresh when caught in distant waters.
[115] Most commercial icemakers produce three basic types of fragmentary ice: flake, tubular and plate, using a variety of techniques.
[119] Whenever there is freezing rain or snow which occurs at a temperature near the melting point, it is common for ice to build up on the windows of vehicles.
[120] A thin layer of ice crystals can also form on the inside surface of car windows during sufficiently cold weather.
It operated in the winters of 1941–1942 and 1942–1943, when it was the only land route available to the Soviet Union to relieve the Siege of Leningrad by the German Army Group North.
Firstly, spray and freezing rain can produce an ice build-up on the superstructure of a vessel sufficient to make it unstable, potentially to the point of capsizing.
[128] Secondly, icebergs – large masses of ice floating in water (typically created when glaciers reach the sea) – can be dangerous if struck by a ship when underway.
In 1919, during the first non-stop flight across the Atlantic, the British aviators Captain John Alcock and Lieutenant Arthur Whitten Brown encountered such icing conditions – Brown left the cockpit and climbed onto the wing several times to remove ice which was covering the engine air intakes of the Vickers Vimy aircraft they were flying.
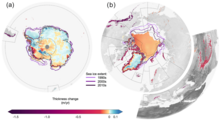
[140] Greenhouse gas emissions from human activities unbalance the Earth's energy budget and so cause an accumulation of heat.
[158][159] The West Antarctic ice sheet is highly vulnerable and will likely disappear even if the warming does not progress further,[164][165][166][167] although it could take around 2,000 years before its loss is complete.