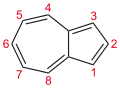
Azulene
Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates.
Azulene has a long history, dating back to the 15th century as the azure-blue chromophore obtained by steam distillation of German chamomile.
It exhibits aromatic properties: (i) the peripheral bonds have similar lengths and (ii) it undergoes Friedel-Crafts-like substitutions.
The dipolar nature of the ground state is reflected in its deep colour, which is unusual for small unsaturated aromatic compounds.
[7] Another notable feature of azulene is that it violates Kasha's rule by exhibiting fluorescence from an upper-excited state (S2 → S0).
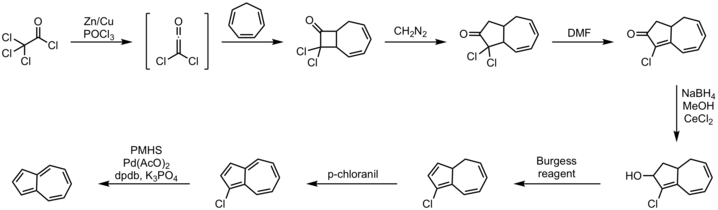
[12][13] Procedure: Another synthesis route starts from the of pyridinium or pyrylium salts with cyclopentadienyl anion:[14] Azulene can also be synthesized via a Diels Alder and retro-Diels Alder reaction:[14] The starting material of the above reaction can be generated through the Flash Vacuum Pyrolysis of phenyl propiolate.