G protein
Some active-state GPCRs have also been shown to be "pre-coupled" with G proteins, whereas in other cases a collision coupling mechanism is thought to occur.
[4][5][6] The G protein triggers a cascade of further signaling events that finally results in a change in cell function.
[7] G proteins regulate metabolic enzymes, ion channels, transporter proteins, and other parts of the cell machinery, controlling transcription, motility, contractility, and secretion, which in turn regulate diverse systemic functions such as embryonic development, learning and memory, and homeostasis.
[8] G proteins were discovered in 1980 when Alfred G. Gilman and Martin Rodbell investigated stimulation of cells by adrenaline.
Prominent examples include (in chronological order of awarding): G proteins are important signal transducing molecules in cells.
"Malfunction of GPCR [G Protein-Coupled Receptor] signaling pathways are involved in many diseases, such as diabetes, blindness, allergies, depression, cardiovascular defects, and certain forms of cancer.
"[15] The human genome encodes roughly 800[16] G protein-coupled receptors, which detect photons of light, hormones, growth factors, drugs, and other endogenous ligands.
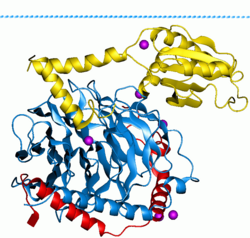
These proteins are homologous to the alpha (α) subunit found in heterotrimers, but are in fact monomeric, consisting of only a single unit.
However, models which suggest molecular rearrangement, reorganization, and pre-complexing of effector molecules are beginning to be accepted.
[24] The Gα subunit will eventually hydrolyze the attached GTP to GDP by its inherent enzymatic activity, allowing it to re-associate with Gβγ and starting a new cycle.
[25] Gαs activates the cAMP-dependent pathway by stimulating the production of cyclic AMP (cAMP) from ATP.
e.g. somatostatin, prostaglandins Gαq/11 stimulates the membrane-bound phospholipase C beta, which then cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into two second messengers, inositol trisphosphate (IP3) and diacylglycerol (DAG).
DAG activates protein kinase C. The Inositol Phospholipid Dependent Pathway is used as a signal transduction pathway for many hormones including: Small GTPases, also known as small G-proteins, bind GTP and GDP likewise, and are involved in signal transduction.