G protein-coupled receptor
The long ago discovered association between GPCRs and many endogenous and exogenous substances, resulting in e.g. analgesia, is another dynamically developing field of the pharmaceutical research.
The way in which the seven transmembrane helices of a GPCR are arranged into a bundle was suspected based on the low-resolution model of frog rhodopsin from cryogenic electron microscopy studies of the two-dimensional crystals.
The crystal structure of rhodopsin, that came up three years later, was not a surprise apart from the presence of an additional cytoplasmic helix H8 and a precise location of a loop covering retinal binding site.
However, it provided a scaffold which was hoped to be a universal template for homology modeling and drug design for other GPCRs – a notion that proved to be too optimistic.
[3] The 2012 Nobel Prize in Chemistry was awarded to Brian Kobilka and Robert Lefkowitz for their work that was "crucial for understanding how G protein-coupled receptors function".
[17] An early study based on available DNA sequence suggested that the human genome encodes roughly 750 G protein-coupled receptors,[18] about 350 of which detect hormones, growth factors, and other endogenous ligands.
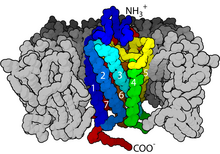
Some examples of their physiological roles include: GPCRs are integral membrane proteins that possess seven membrane-spanning domains or transmembrane helices.
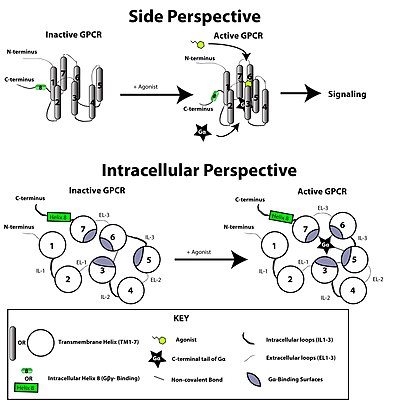
The GPCR arranges itself into a tertiary structure resembling a barrel, with the seven transmembrane helices forming a cavity within the plasma membrane that serves a ligand-binding domain that is often covered by EL-2.
Upon glutamate-binding to an mGluR, the N-terminal tail undergoes a conformational change that leads to its interaction with the residues of the extracellular loops and TM domains.
Inverse agonists and antagonists may also bind to a number of different sites, but the eventual effect must be prevention of this TM helix reorientation.
[37] In particular, the C-terminus often contains serine (Ser) or threonine (Thr) residues that, when phosphorylated, increase the affinity of the intracellular surface for the binding of scaffolding proteins called β-arrestins (β-arr).
[38] Once bound, β-arrestins both sterically prevent G-protein coupling and may recruit other proteins, leading to the creation of signaling complexes involved in extracellular-signal regulated kinase (ERK) pathway activation or receptor endocytosis (internalization).
As the phosphorylation of these Ser and Thr residues often occurs as a result of GPCR activation, the β-arr-mediated G-protein-decoupling and internalization of GPCRs are important mechanisms of desensitization.
Palmitoylation is the covalent modification of cysteine (Cys) residues via addition of hydrophobic acyl groups, and has the effect of targeting the receptor to cholesterol- and sphingolipid-rich microdomains of the plasma membrane called lipid rafts.
As many of the downstream transducer and effector molecules of GPCRs (including those involved in negative feedback pathways) are also targeted to lipid rafts, this has the effect of facilitating rapid receptor signaling.
This is made possible by a guanine-nucleotide exchange factor (GEF) domain primarily formed by a combination of IL-2 and IL-3 along with adjacent residues of the associated TM helices.
GPCRs include one or more receptors for the following ligands: sensory signal mediators (e.g., light and olfactory stimulatory molecules); adenosine, bombesin, bradykinin, endothelin, γ-aminobutyric acid (GABA), hepatocyte growth factor (HGF), melanocortins, neuropeptide Y, opioid peptides, opsins, somatostatin, GH, tachykinins, members of the vasoactive intestinal peptide family, and vasopressin; biogenic amines (e.g., dopamine, epinephrine, norepinephrine, histamine, serotonin, and melatonin); glutamate (metabotropic effect); glucagon; acetylcholine (muscarinic effect); chemokines; lipid mediators of inflammation (e.g., prostaglandins, prostanoids, platelet-activating factor, and leukotrienes); peptide hormones (e.g., calcitonin, C5a anaphylatoxin, follicle-stimulating hormone [FSH], gonadotropin-releasing hormone [GnRH], neurokinin, thyrotropin-releasing hormone [TRH], and oxytocin); and endocannabinoids.
At this point, the subunits of the G-protein dissociate from the receptor, as well as each other, to yield a Gα-GTP monomer and a tightly interacting Gβγ dimer, which are now free to modulate the activity of other intracellular proteins.
The extent to which they may diffuse, however, is limited due to the palmitoylation of Gα and the presence of an isoprenoid moiety that has been covalently added to the C-termini of Gγ.
Because the signal transducing properties of the various possible βγ combinations do not appear to radically differ from one another, these classes are defined according to the isoform of their α-subunit.
When the subtype activated depends on the ligand that is bound to the GPCR, this is called functional selectivity (also known as agonist-directed trafficking, or conformation-specific agonism).
Regardless of these various nuances, the GPCR's preferred coupling partner is usually defined according to the G-protein most obviously activated by the endogenous ligand under most physiological or experimental conditions.
The presence of a tyrosine-phosphorylated ITIM (immunoreceptor tyrosine-based inhibitory motif) sequence in the B2 receptor is necessary to mediate this interaction and subsequently the antiproliferative effect of bradykinin.
There may even be specific proteins of these classes whose primary function is as part of GPCR-independent pathways, termed activators of G-protein signalling (AGS).
[5] The cAMP signal transduction contains five main characters: stimulative hormone receptor (Rs) or inhibitory hormone receptor (Ri); stimulative regulative G-protein (Gs) or inhibitory regulative G-protein (Gi); adenylyl cyclase; protein kinase A (PKA); and cAMP phosphodiesterase.
At this point, the adapter molecules and clathrin have dissociated, and the receptor is either trafficked back to the plasma membrane or targeted to lysosomes for degradation.
At any point in this process, the β-arrestins may also recruit other proteins—such as the non-receptor tyrosine kinase (nRTK), c-SRC—which may activate ERK1/2, or other mitogen-activated protein kinase (MAPK) signaling through, for example, phosphorylation of the small GTPase, Ras, or recruit the proteins of the ERK cascade directly (i.e., Raf-1, MEK, ERK-1/2) at which point signaling is initiated due to their close proximity to one another.
Dynamins polymerize around the neck of an incoming vesicle, and their phosphorylation by c-SRC provides the energy necessary for the conformational change allowing the final "pinching off" from the membrane.
In addition, lysosomes contain many degradative enzymes, including proteases, which can function only at such low pH, and so the peptide bonds joining the residues of the GPCR together may be cleaved.
[62] Characteristic motifs indicate that three of the five GRAFS families, Rhodopsin, Adhesion, and Frizzled, evolved from the Dictyostelium discoideum cAMP receptors before the split of opisthokonts.