γ-Hydroxybutyric acid
[17] The authors of a 2010 Cochrane review[18] concluded that "GHB appears better than NTX and disulfiram in maintaining abstinence and preventing craving in the medium term (3 to 12 months)".
Its favorable safety profile relative to ethanol may explain why GHB continues to be investigated as a candidate for alcohol substitution.
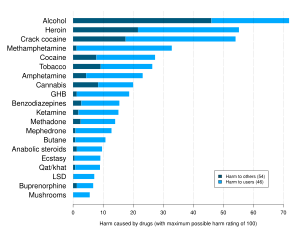
[30] At higher doses, GHB may induce nausea, dizziness, drowsiness, agitation, visual disturbances, depressed breathing, amnesia, unconsciousness, and death.
Co-administration with other CNS depressants such as alcohol or benzodiazepines can result in an additive effect (potentiation), as they all bind to gamma-aminobutyric acid (or "GABA") receptor sites.
[31] Consuming GHB with alcohol can cause respiratory arrest and vomiting in combination with unarousable sleep, which can lead to death.
[35] GBL and 1,4-B are normally found as pure liquids, but they can be mixed with other more harmful solvents when intended for industrial use (e.g. as paint stripper or varnish thinner).
[42][46][47] More recently, a study in Western Australia reviewed the pre-hospital context given in medical records around emergency department presentations with analytical confirmation of GHB exposure.
This study found that most cases reported daily dosing and subsequent accidental overdose rather than their presentation being associated with date-rape.
[48] There have been several high-profile cases of GHB as a date-rape drug that received national attention in the United States.
[49] In the United Kingdom, British serial killer Stephen Port administered GHB to his victims by adding it to drinks given to them, raping them, and murdering four of them in his flat in Barking, East London.
[55] A review of the details of 194 deaths attributed to or related to GHB over a ten-year period found that most were from respiratory depression caused by interaction with alcohol or other drugs.
[29][70][71] The withdrawal syndrome can be severe producing acute delirium and may require hospitalization in an intensive care unit for management.
A newer synthetic drug, SCH-50911, which acts as a selective GABAB antagonist, quickly reverses GHB overdose in mice.
[9] GHB may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized patients,[29] to provide evidence in an impaired driving, or to assist in a medicolegal death investigation.
Blood or plasma GHB concentrations are usually in a range of 50–250 mg/L in persons receiving the drug therapeutically (during general anesthesia), 30–100 mg/L in those arrested for impaired driving, 50–500 mg/L in acutely intoxicated patients and 100–1000 mg/L in victims of fatal overdosage.
[87] People with the disorder known as succinic semialdehyde dehydrogenase deficiency, also known as γ-hydroxybutyric aciduria, have elevated levels of GHB in their urine, blood plasma and cerebrospinal fluid.
[92] In spite of its demonstrated neurotoxicity, (see relevant section, above), GHB has neuroprotective properties, and has been found to protect cells from hypoxia.
GHB-induced stimulation of tissue serotonin turnover may be due to an increase in tryptophan transport to the brain and in its uptake by serotonergic cells.
[103] However, at therapeutic doses, GHB reaches much higher concentrations in the brain and activates GABAB receptors, which are primarily responsible for its sedative effects.
Of these analogues, only 4-methyl-GHB (γ-hydroxyvaleric acid, GHV) and a prodrug form γ-valerolactone (GVL) have been reported as drugs of abuse in humans, and on the available evidence seem to be less potent but more toxic than GHB, with a particular tendency to cause nausea and vomiting.
[120]: 11–12 [121] It was studied in a range of uses including obstetric surgery and during childbirth and as an anxiolytic; there were anecdotal reports of it having antidepressant and aphrodisiac effects as well.
[120]: 28 In May 1990 GHB was introduced as a dietary supplement and was marketed to body builders, for help with weight control and as a sleep aid, and as a "replacement" for l-tryptophan, which was removed from the market in November 1989 when batches contaminated with trace impurities[123] were found to cause eosinophilia–myalgia syndrome, although eosinophilia–myalgia syndrome is also tied to tryptophan overload.
[128][129][130] At the same time, research on the use of GHB in the form of sodium oxybate had formalized, as a company called Orphan Medical had filed an investigational new drug application and was running clinical trials with the intention of gaining regulatory approval for use to treat narcolepsy.
[120]: 18–25, 28 [131]: 10 A popular children's toy, Bindeez (also known as Aqua Dots, in the United States), produced by Melbourne company Moose, was banned in Australia in early November 2007 when it was discovered that 1,4-butanediol (1,4-B), which is metabolized into GHB, had been substituted for the non-toxic plasticiser 1,5-pentanediol in the bead manufacturing process.
Possession of the substance for consumption without license from the Department of Health is illegal with a HK$100,000 fine or five years of jail time.
[139] In New Zealand and Australia, GHB, 1,4-B, and GBL are all Class B illegal drugs, along with any possible esters, ethers, and aldehydes.
GABA itself is also listed as an illegal drug in these jurisdictions, which seems unusual given its failure to cross the blood–brain barrier, but there was a perception among legislators that all known analogues should be covered as far as this was possible.
Attempts to circumvent the illegal status of GHB have led to the sale of derivatives such as 4-methyl-GHB (γ-hydroxyvaleric acid, GHV) and its prodrug form γ-valerolactone (GVL), but these are also covered under the law by virtue of their being "substantially similar" to GHB or GBL, so importation, sale, possession and use of these compounds is also considered to be illegal.
In Chile, GHB is a controlled drug under the law Ley de substancias psicotrópicas y estupefacientes (psychotropic substances and narcotics).
In Norway[140] and in Switzerland,[141] GHB is considered a narcotic and is only available by prescription under the trade name Xyrem (Union Chimique Belge S.A.).