Gas thermometer
Charles's Law states that when the temperature of a gas increases, so does the volume.
Translating it to the correct levels of the device that is holding the gas.
The constant volume gas thermometer plays a crucial role in understanding how absolute zero could be discovered long before the advent of cryogenics.
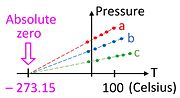
Consider a graph of pressure versus temperature made not far from standard conditions (well above absolute zero) for three different samples of any ideal gas (a, b, c).
[3] Note that data could have been collected with three different amounts of the same gas, which would have rendered this experiment easy to do in the eighteenth century.