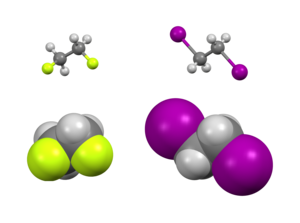Gauche effect
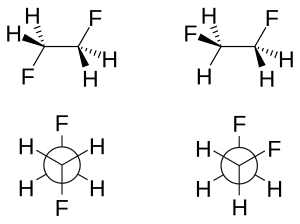
Typically studied examples include 1,2-difluoroethane (H2FCCFH2), ethylene glycol, and vicinal-difluoroalkyl structures.
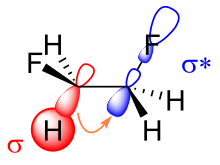
The resulting reduced orbital overlap can be partially compensated when a gauche conformation is assumed, forming a bent bond.
[5][6] The molecular geometry of both rotamers can be obtained experimentally by high-resolution infrared spectroscopy augmented with in silico work.
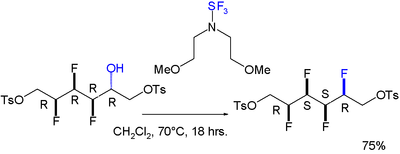
A gauche effect has also been reported for a molecule featuring an all-syn array of four consecutive fluoro substituents.
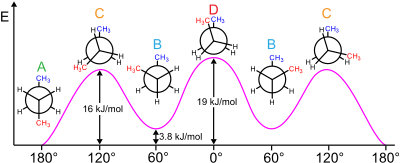
The reaction to install the fourth one is stereoselective:[8] The gauche effect is also seen in 1,2-dimethoxyethane[citation needed] and some vicinal-dinitroalkyl compounds.