Anomeric effect
In the tetrahydropyran ring, Y = oxygen, which is a heteroatom, so the anomeric effect contributes and stabilizes the observed substituent position.
The anomeric effect is most often observed when Y = oxygen, but can also be seen with other lone pair bearing heteroatoms in the ring, such as nitrogen, sulfur, and phosphorus.
[4] The exact method by which the anomeric effect causes stabilization is a point of controversy, and several hypotheses have been proposed to explain it.
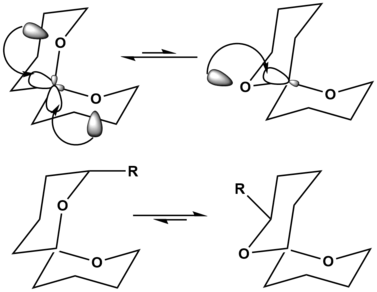
[5] A widely accepted explanation is that there is a stabilizing interaction (hyperconjugation) between the unshared electron pair on the endocyclic heteroatom (within the sugar ring) and the σ* orbital of the axial (exocyclic) C–X bond.
[7] Some authors also question the validity of this hyperconjugation model based on results from the quantum theory of atoms in molecules.
[8] While most studies on the anomeric effects have been theoretical in nature, the n–σ* (hyperconjugation) hypothesis has also been extensively criticized on the basis that the electron density redistribution in acetals proposed by this hypothesis is not congruent with the known experimental chemistry of acetals and, in particular, the chemistry of monosaccharides.
[11] Another accepted explanation for the anomeric effect is the equatorial configuration has the dipoles involving both heteroatoms partially aligned, and therefore repelling each other.
[12] By contrast the axial configuration has these dipoles roughly opposing, thus representing a more stable and lower energy state.
In the (Z) conformation the lone pair of electrons in the alpha oxygen can donate into the neighboring σ* C-O orbital.
[3] One common criticism of the hyperconjugation theory is that it fails to explain why the anomeric effect is not observed when substituted tetrahydropyran molecules are placed in polar solvents, and the equatorial position is once again preferred.
The orientation on the upper right only shows this hyperconjugative anomeric stabilization once, causing it to be the lesser preferred structure.
As the anomeric effect predicts, the methoxy substituent shows an increased preference for the axial conformation.
[15] This term refers to the apparent preference of positively charged nitrogen substituents for the equatorial conformation beyond what normal steric interactions would predict in rings containing an electronegative atom, such as oxygen.
Late transition metals from groups 10, 11, and 12 when placed at the anomeric carbon show strong axial preferences.
[19] This phenomenon termed as the metallo-anomeric effect originates from stabilizing hyperconjugative interactions between oxygen or other heteroatoms with lone pairs and C-M anti-bonding orbitals that act as good acceptors.
In general terms, moving from a lighter to a heavier element in the group, the magnitude of the metallo-anomeric effect increases.