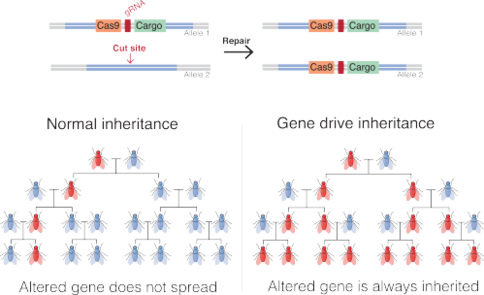
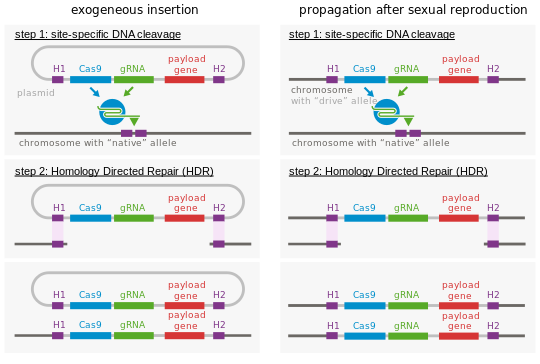
Gene drive
Any accidental return of individuals of the species to its original habitats, through natural migration, environmental disruption (storms, floods, etc.
Cells are frequently co-infected by multiple virions and recombination between viral genomes is a well-known and widespread source of diversity for many viruses.
These properties have enabled the design of a gene drive strategy that doesn't involve sexual reproduction, instead relying on co-infection of a given cell by a naturally occurring and an engineered virus.
Upon co-infection, the unmodified genome is cut and repaired by homologous recombination, producing new gene drive viruses that can progressively replace the naturally occurring population.
[19] Issues highlighted by researchers include:[20] The Broad Institute of MIT and Harvard added gene drives to a list of uses of gene-editing technology it doesn't think companies should pursue.
[25][26] In June 2016, the US National Academies of Sciences, Engineering, and Medicine released a report on their "Recommendations for Responsible Conduct" of gene drives.
Accordingly, they should only be built to combat true plagues such as malaria, for which we have few adequate countermeasures and that offer a realistic path towards an international agreement to deploy among all affected nations.".
[29] He moved to an open model for his own research on using gene drives to eradicate Lyme disease in Nantucket and Martha's Vineyard.
Esvelt later retracted his support for the idea, except for extremely hazardous populations such as malaria-carrying mosquitoes, and isolated islands that would prevent the drive from spreading beyond the target area.
[31] Austin Burt, an evolutionary geneticist at Imperial College London, introduced the possibility of conducting gene drives based on natural homing endonuclease selfish genetic elements in 2003.
Gene drives based on homing endonucleases have been demonstrated in the laboratory in transgenic populations of mosquitoes[32] and fruit flies.
In June 2014, the World Health Organization (WHO) Special Programme for Research and Training in Tropical Diseases[35] issued guidelines[36] for evaluating genetically modified mosquitoes.
Target Malaria, a project funded by the Bill and Melinda Gates Foundation, invested $75 million in gene drive technology.
[38] In December 2017, documents released under the Freedom of Information Act showed that DARPA had invested $100 million in gene drive research.
The paper's senior author cautions that the two neutralizing systems they demonstrated in cage trials "should not be used with a false sense of security for field-implemented gene drives".
[40][41] If elimination is not necessary, it may be desirable to intentionally preserve the target population at a lower level by using a less severe gene drive technology.
[47][48] They reported efficient inheritance distortion over successive generations, with one study demonstrating the spread of a gene into laboratory populations.
[49] Gene drives have two main classes of application, which have implications of different significance: Because of their unprecedented potential risk, safeguard mechanisms have been proposed and tested.
Given the risks of such an approach described below, the GBIRd partnership is committed to a deliberate, step-wise process that will only proceed with public alignment, as recommended by the world's leading gene drive researchers from the Australian and US National Academy of Sciences and many others.
[61] In 2017, scientists at the University of California, Riverside developed a gene drive to attack the invasive spotted-wing drosophila, a type of fruit fly native to Asia that is costing California's cherry farms $700 million per year because of its tail's razor-edged ovipositor that destroys unblemished fruit.
[21] The transhumanist philosopher David Pearce has advocated for using CRISPR-based gene drives to reduce the suffering of wild animals.