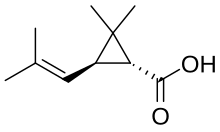
Pyrethroid
A pyrethroid is an organic compound similar to the natural pyrethrins, which are produced by the flowers of pyrethrums (Chrysanthemum cinerariaefolium and C. coccineum).
[1] However, pyrethroids are toxic to insects such as bees, dragonflies, mayflies, gadflies, and some other invertebrates, including those that constitute the base of aquatic and terrestrial food webs.
This interior is shaped precisely to allow sodium ions to pass through the membrane, enter the axon, and propagate an action potential.
When the toxin keeps the channels in their open state, the nerves cannot repolarize, leaving the axonal membrane permanently depolarized, thereby paralyzing the organism.
[8] Pyrethroids are toxic to insects such as bees, dragonflies, mayflies, gadflies, and some other invertebrates, including those that constitute the base of aquatic and terrestrial food webs.
Typical symptoms include facial paresthesia, itching, burning, dizziness, nausea, vomiting and more severe cases of muscle twitching.
Severe poisoning is often caused by ingestion of pyrethroids and can result in a variety of symptoms like seizures, coma, bleeding or pulmonary edema.
[27] Pyrethroids were introduced by a team of Rothamsted Research scientists in the 1960s and 1970s following the elucidation of the structures of pyrethrin I and II by Hermann Staudinger and Leopold Ružička in the 1920s.
Their work consisted firstly of identifying the most active components of pyrethrum, extracted from East African chrysanthemum flowers and long known to have insecticidal properties.
Pyrethrum rapidly knocks down flying insects but has negligible persistence — which is good for the environment but gives poor efficacy when applied in the field.
Pyrethroids are essentially chemically stabilized forms of natural pyrethrum and belong to IRAC MoA group 3 (they interfere with sodium transport in insect nerve cells).
With the 91/414/EEC review,[30] many 1st-generation compounds have not been included on Annex 1, probably because the market is not big enough to warrant the costs of re-registration (rather than any special concerns about safety).
They are substantially more resistant to degradation by light and air, thus making them suitable for use in agriculture, but they have significantly higher mammalian toxicities.