Genotoxicity
Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer.
[1] Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis.
This DNA damage can be in the form of single- and double-strand breaks, loss of excision repair, cross-linking, alkali-labile sites, point mutations, and structural and numerical chromosomal aberrations.
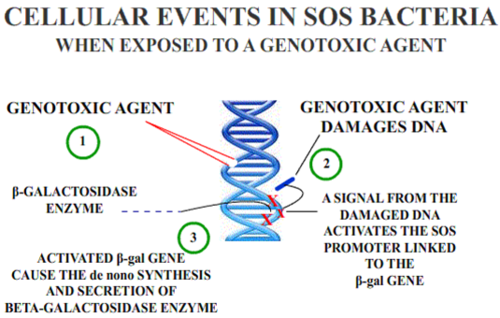
Genotoxic substances induce damage to the genetic material in the cells through interactions with the DNA sequence and structure.
Researchers performed an experiment to study the interaction between DNA with the carcinogenic chromium by using a Cr(V)-Salen complex at the specific oxidation state.
High-valent chromium is seen to act as a carcinogen as researchers found that "the mechanism of damage and base oxidation products for the interaction between high-valent chromium and DNA... are relevant to in vivo formation of DNA damage leading to cancer in chromate-exposed human populations".
These substances are found mainly in plant species and are poisonous to animals, including humans; about half of them have been identified as genotoxic and many as tumorigenic.
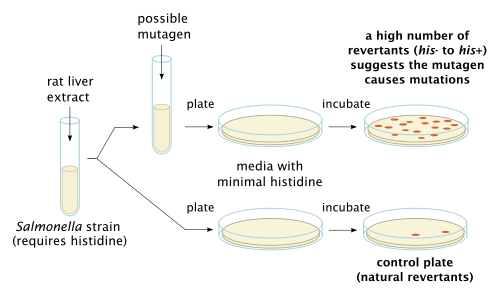
[6] The purpose of in vitro testing is to determine whether a substrate, product, or environmental factor induces genetic damage.
The clastogenic or aneugenic effects from the genotoxic damage will cause an increase in frequency of structural or numerical aberrations of the genetic material.
[1] In a specific mammalian tissue, one can perform a mouse lymphoma TK+/- assay to test for changes in the genetic material.
The specific type of damage is determined by the size of the colonies, distinguishing between genetic mutations (mutagens) and chromosomal aberrations (clastogens).
The DNA released from the lysed cell is electrophoresed in an agarose gel under neutral pH conditions.
Some chemicals have the ability to induce fragile sites in regions of the chromosome where oncogenes are present, which could lead to carcinogenic effects.
In keeping with this finding, occupational exposure to some mixtures of pesticides are positively correlated with increased genotoxic damage in the exposed individuals.
The difference in ability to detoxify certain compounds is due to individuals' inherited polymorphisms of genes involved in the metabolism of the chemical.
[11] Major genotoxic agents responsible for the four most common cancers worldwide (lung, breast, colon and stomach) have been identified.
[13] By this approach, the tumorigenic compounds in tobacco smoke were, in order of importance, acrolein, formaldehyde, acrylonitrile, 1,3-butadiene, cadmium, acetaldehyde, ethylene oxide, and isoprene.
[17] Estrogen likely contributes to breast carcinogenesis by the following three processes; (1) metabolic conversion of estrogen to genotoxic, mutagenic carcinogens, (2) stimulation of growth of tissues, and (3) repression of phase II detoxification enzymes that metabolize genotoxic reactive oxygen species, thus resulting in increased oxidative DNA damage.
[22] In addition, bile acids are implicated by substantial evidence as an important genotoxic factor in colon cancer.
H. pylori infection of gastric epithelial cells causes increased production of genotoxic reactive oxygen species (ROS).
By utilizing the destructive properties of genotoxins treatments aims to induce DNA damage into cancer cells.