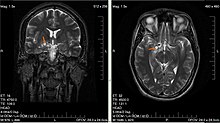
Glioma
A brain glioma can cause headaches, vomiting, seizures, and cranial nerve disorders as a result of increased intracranial pressure.
[12] It was considered possible,[13][14] though several large studies have found no conclusive evidence, as summarized by the NIH's National Cancer Institute review of the topic[15] and its numerous citations,[16] and the FCC.
[17] However, further research is still being pursued to obtain more robust evidence and verify that there is no relationship (the NIH's National Institute of Environmental Health Sciences most recent press release discussed an ongoing study[18] showing mildly positive results,[19] although it appears there may have been issues with the control group dying prematurely[20]).
[21][22][23] However, this is a controversial opinion, with recent in-depth studies failing to find an association between viral infection and glioma growth.
In a 2021 meta-analysis, 40 of 52 studies since 1998 reported positive associations between farming and brain cancer with effect estimates ranging from 1.03 to 6.53, of which 80% are gliomas.
Farmers with documented exposure to pesticides had greater than a 20% elevated risk of brain cancer[26][unreliable source?]
[28] Germ-line (inherited) polymorphisms of the DNA repair genes ERCC1, ERCC2 (XPD) and XRCC1 increase the risk of glioma.
[31][32] Such mutations and epimutations may provide a cell with a proliferative advantage which can then, by a process of natural selection, lead to progression to cancer.
[43] Wang et al.[44] pointed out that IDH1 and IDH2 mutant cells produce an excess metabolic intermediate, 2-hydroxyglutarate, which binds to catalytic sites in key enzymes that are important in altering histone and DNA promoter methylation.
As a rule, high-grade gliomas almost always grow back even after complete surgical excision, so are commonly called recurrent cancer of the brain.
[medical citation needed] Several acquired (not inherited) genetic mutations have been found in gliomas.
[52] Gliomas are named according to the specific type of cell with which they share histological features, but not necessarily from which they originate.
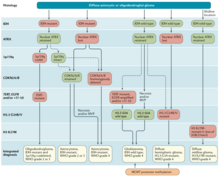
The neuropathological evaluation and diagnostics of brain tumor specimens is performed according to WHO Classification of Tumours of the Central Nervous System.
The modern approach to the diagnosis of diffuse gliomas takes mainly the histopathology and molecular profile into account.
[61] Tissue specimens obtained through biopsy sampling in patients with diffuse gliomas are routinely assessed by immunohistochemistry for the presence of R132H-mutant IDH1 and loss of nuclear ATRX.
[61] In all other instances of diffuse gliomas, a lack of IDH1 R132H immunopositivity should be followed by IDH1 and IDH2 DNA sequencing to detect or exclude the presence of non-canonical mutations.
Temozolomide is a common chemotherapy drug which can be administered easily in an outpatient setting and is able to cross the blood–brain barrier effectively.
There are a wide variety of novel treatments currently being tested in clinical trials, ranging from IDH inhibitors like Ivosidenib, to the recently approved Dendritic cell-based cancer vaccine approach.
[68][69] Experimental therapies like oncolytic viruses have shown potential therapeutic benefits in clinical trials (but have not been approved for use in non-experimental settings).
[70] For recurrent high-grade glioblastoma, recent studies have taken advantage of angiogenic blockers such as bevacizumab in combination with conventional chemotherapy, with encouraging results.
[72] The relative effectiveness of surgical resection compared to biopsy for people with malignant glioma (high-grade) is unknown.
[74] A 2019 meta-analysis suggested that for people with less aggressive gliomas, radiotherapy may increase the risk of long term neurocognitive side effects.
[67] A 2013 meta-analysis showed that Temozolomide prolongs survival and delays progression, but is associated with an increase in side effects such as blood complications, fatigue, and infection.
[76] A mutational analysis of 23 initial low-grade gliomas and recurrent tumors from the same patients has challenged the benefits and usage of Temozolomide.
Prognosis of gliomas is given in relation to what grade (as scored by the World Health Organization system) of tumour the patient presents with.
[84] Unfortunately, approximately 70% of low-grade (WHO grade-II) will progress to high-grade tumours within 5–10 years[49] Grade II gliomas, despite often being labeled as benign, are considered a uniformly fatal illness.
Hypofractionated radiation therapy has similar efficacy for survival as compared to conventional radiotherapy, particularly for individuals aged 60 and older with glioblastoma.
By their very nature, these tumours invade diffusely throughout the brain stem, growing between normal nerve cells.