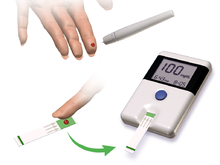
Glucose meter
[2][3] In 1962, Clark and Ann Lyons from the Cincinnati Children's Hospital developed the first glucose enzyme electrode.
The two models initially dominant in North America in the 1980s were the Glucometer, introduced in November 1981,[4] whose trademark is owned by Bayer, and the Accu-Chek meter (by Roche).
BM stands for Boehringer Mannheim, now part of Roche, who produce test strips called 'BM-test' for use in a meter.
[5][6] In North America, hospitals resisted adoption of meter glucose measurements for inpatient diabetes care for over a decade.
[citation needed] Prior to its discontinuation in July 2021, the YSI 2300 STAT PLUS Glucose and Lactate Analyzer was widely accepted as the de facto standard for reference measurements and system calibration by most manufacturers of glucometers for the past 30 years, despite there being no such regulatory requirement.
The manufacturer cited studies that show the product is just as effective despite not giving an answer to one decimal place, something they argue is unnecessary for control of blood sugar.
On May 1, 2009, the UK distributor Ambe Medical Group reduced the price of their "Glucoflex-R" test strip to the NHS, by approximately 50%.
Their data handling capabilities are designed to transfer glucose results into electronic medical records and the laboratory computer systems for billing purposes.
There are several key characteristics of glucose meters which may differ from model to model: Countries that use mmol/L include Australia, Canada, China, Croatia, Czech Republic, Denmark, Finland, Hong Kong, Hungary, Iceland, Ireland, Jamaica, Kazakhstan, Latvia, Lithuania, Malaysia, Malta, Netherlands, New Zealand, Norway, Russia, Slovakia, Slovenia, South Africa, Sweden, Switzerland, and United Kingdom.
[14][15] Countries that use mg/dL include Algeria, Argentina, Austria, Bangladesh, Belgium, Brazil, Chile, Columbia, Cyprus, Ecuador, Egypt, France, Georgia, Germany, Greece, India, Indonesia, Iran, Israel, Italy, Japan, Jordan, Korea, Lebanon, Mexico, Peru, Poland, Portugal, South Korea, Spain, Syria, Taiwan, Thailand, Tunisia, Turkey, United Arab Emirates, United States, Uruguay, Venezuela, and Yemen.
In the UK, where the National Health Service (NHS) rather than patients pay for medications including test strips, a 2015 study on the comparative cost-effectiveness of all options for the self-monitoring of blood glucose funded by the NHS uncovered considerable variation in the price charged, which could not be explained by the availability of advanced meter features.
It estimated that a total of £12m was invested in providing 42 million self-monitoring blood glucose tests with systems that failed to meet acceptable accuracy standards, and efficiency savings of £23.2m per annum were achievable if the NHS were to disinvest from technologies providing less functionality than available alternatives, but at a much higher price.
Models vary in their susceptibility to these factors and in their ability to prevent or warn of inaccurate results with error messages.
The Clarke Error Grid has been a common way of analyzing and displaying accuracy of readings related to management consequences.
[28][29][30] Apple has patented methods for determining blood sugar levels by absorption spectroscopy, as well as by analyzing exhaled air in its electronic devices.
The disadvantage of this method was that the test strip had to be developed after a precise interval (the blood had to be washed away), and the meter needed to be calibrated frequently.
The enzyme is reoxidized with an excess of a mediator reagent, such as a ferricyanide ion, a ferrocene derivative or osmium bipyridyl complex.
This is to allow for continuous regeneration of the oxidised form of the mediator for shuttling of electrons from enzyme to active site.
[32][33] The total charge passing through the electrode is proportional to the amount of glucose in the blood that has reacted with the enzyme.
The coulometric method is a technique where the total amount of charge generated by the glucose oxidation reaction is measured over a period of time.
The amperometric method is used by some meters and measures the electric current generated at a specific point in time by the glucose reaction.
People with type 1 diabetes usually have a wider range of glucose levels, and glucose peaks above normal, often ranging from 40 to 500 mg/dL (2.2 to 28 mmol/L), and when a meter reading of 50 or 70 (2.8 or 3.9 mmol/L) is accompanied by their usual hypoglycemic symptoms, there is little uncertainty about the reading representing a "true positive" and little harm done if it is a "false positive."
However, the incidence of hypoglycemia unawareness, hypoglycemia-associated autonomic failure (HAAF) and faulty counterregulatory response to hypoglycemia make the need for greater reliability at low levels particularly urgent in patients with type 1 diabetes mellitus, while this is seldom an issue in the more common form of the disease, type 2 diabetes mellitus.
In contrast, people who do not have diabetes may periodically have hypoglycemic symptoms but may also have a much higher rate of false positives to true, and a meter is not accurate enough to base a diagnosis of hypoglycemia upon.
A meter can occasionally be useful in the monitoring of severe types of hypoglycemia (e.g., congenital hyperinsulinism) to ensure that the average glucose when fasting remains above 70 mg/dL (3.9 mmol/L).