Glycerol dehydrogenase
[1][2] This enzyme is an oxidoreductase, specifically a metal-dependent alcohol dehydrogenase that plays a role in anaerobic glycerol metabolism and has been isolated from a number of bacteria, including Enterobacter aerogenes,[3] Klebsiella aerogenes,[4] Streptococcus faecalis,[5] Erwinia aeroidea,[6] Bacillus megaterium,[7] and Bacillus stearothermophilus.
[8] Glycerol dehydrogenase is a homooctamer composed of eight identical monomer subunits made up of a single polypeptide chain of 370 amino acids (molecular weight 42,000 Da).
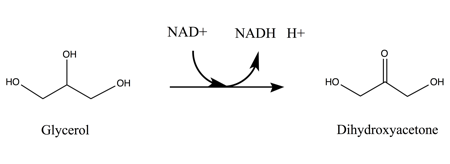
[10] While the precise mechanism of this specific enzyme has not yet been characterized, kinetic studies support that GlyDH catalysis of the chemical reaction is comparable to those of other alcohol dehydrogenases.
After NAD+ is bound to the enzyme, glycerol substrate binds to the active site in such a way as to have two coordinated interactions between two adjacent hydroxyl groups and the neighboring zinc ion.
Biotechnology is one such technique: using particular enzymes to break down crude glycerol to form products such as 1,3-propanediol, 1,2-propanediol, succinic acid, dihydroxyacetone (glycerone), hydrogen, polyglycerols, and polyesters.