Lactoylglutathione lyase
The enzyme lactoylglutathione lyase (EC 4.4.1.5, also known as glyoxalase I) catalyzes the isomerization of hemithioacetal adducts, which are formed in a spontaneous reaction between a glutathionyl group and aldehydes such as methylglyoxal.
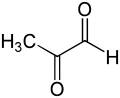
Methylglyoxal is produced naturally as a byproduct of normal biochemistry, but is highly toxic, due to its chemical reactions with proteins, nucleic acids, and other cellular components.
Unusually, these reactions carried out by the glyoxalase system does not oxidize glutathione, which usually acts as a redox coenzyme.
Structurally, the enzyme is a domain-swapped dimer in many species, although the two subunits have merged into a monomer in yeast, through gene duplication.
However, in yeast such as Saccharomyces cerevisiae, the two subunits have fused into a single monomer of double size, through gene duplication.
[1] Finally, many proteins of unknown or uncertain function likewise resemble glyoxalase I, such as At5g48480 from the plant, Arabidopsis thaliana.
[11] Methylglyoxal is a by-product of normal biochemistry that is a carcinogen, a mutagen[12] and can chemically damage several components of the cell, such as proteins and nucleic acids.
For example, oxidoreductases often use a specific metal ion such as iron, manganese or copper and will fail to function if their preferred metal ion is replaced, due to differences in the redox potential; thus, the ferrous superoxide dismutase cannot function if its catalytic iron is replaced by manganese, and vice versa.
[24] Similarly, although the prokaryotic glyoxalase I prefers nickel, it is able to function with cobalt, manganese and cadmium; however, the enzyme is inert with bound zinc, due to a change in coordination geometry from octahedral to trigonal bipyramidal.
[15] Structural and computational studies have revealed that the metal binds the two carbonyl oxygens of the methylglyoxal moiety at two of its coordination sites, stabilizing the enediolate anion intermediate.
[26] The catalytic mechanism of Glyoxalase have been studied by density functional theory, molecular dynamics simulations and hybrid QM/MM methods.
At the same time, the extra electron on the oxygen of C1 could reform the double bond of the carbonyl, thus giving the final product.
The ene means that a double bond has formed between C2 and C1, from the electrons left behind by the abstraction of the aldehyde proton; the diol refers to the fact that two alcohols have been made of the initial two carbonyl groups.
However, an alternate hypothesis — that the enzyme active site was deeply buried away from water — could not be ruled out and ultimately proved to be correct.
The clinching evidence can with studies of the hydrogen-deuterium isotope effect on substrates fluorinated on the methyl group and deuterated on the aldehyde.
Experiments on three types of glyoxalase I (yeast, rat and mouse forms) supported the proton-transfer mechanism in every case.
[37] It has been proposed that the behavioral effects of Glo1 are due to the activity of its principal substrate methylglyoxal at GABAA receptors.
[38] Glyoxalase I is a target for the development of pharmaceuticals against bacteria, protozoans (especially Trypanosoma cruzi and the Leishmania) and human cancer.
[40] The closest analog of the transition state is believed to be S-(N-hydroxy-N-p-iodophenylcarbamoyl)glutathione; the crystal structure of this compound bound to the human enzyme has been solved to 2 Å resolution (PDB accession code 1QIN).
[42] Recent research demonstrates that GLO1 expression is upregulated in various human malignant tumors including metastatic melanoma.