Grob fragmentation
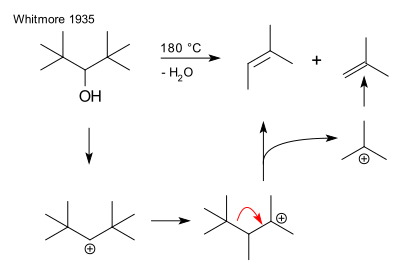
An early instance of fragmentation is the dehydration of di(tert-butyl)methanol yielding 2-methyl-2-butene and isobutene, a reaction described in 1933 by Frank C.
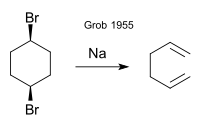
[4] This reaction proceeds by formation of a secondary carbocation followed by a rearrangement reaction to a more stable tertiary carbocation and elimination of a t-butyl cation: Albert Eschenmoser in 1952 investigated the base catalysed fragmentation of certain beta hydroxy ketones:[5] The original work by Grob (1955) concerns the formation of 1,5-hexadiene from cis- or trans-1,4-dibromocyclohexane by sodium metal:[1] According to reviewers Prantz and Mulzer (2010), the name Grob fragmentation was chosen "in more or less glaring disregard of the earlier contributions".
The carbanionic pathway is more common and is facilitated by the stability of the cation formed and the leaving group ability of the nucleofuge.
The selectivity of the initial reduction of ketone 1 is a result of borohyride approaching from the bottom face to avoid steric clash with the axial methyl group.
The reaction mechanism has been reported to begin with the reduction of an ether protected amide to form a secondary alcohol.