Gyrification
The neurons of the cerebral cortex reside in a thin layer of gray matter, only 2–4 mm thick, at the surface of the brain.
[2] Much of the interior volume is occupied by white matter, which consists of long axonal projections to and from the cortical neurons residing near the surface.
Gyrification allows a larger cortical surface area, and hence greater cognitive functionality to fit inside a smaller cranium.
Primates, cetaceans, and ungulates have extensive cortical gyri, with a few species exceptions, while small rodents such as the rat, and mouse have none.
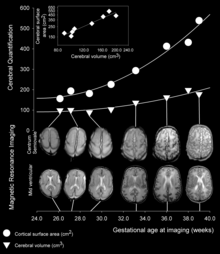
[3] As fetal development proceeds, gyri and sulci begin to take shape with the emergence of deepening indentations on the surface of the cortex.
[6] There is evidence to suggest a positive relationship between gyrification and cognitive information processing speed, as well as better verbal working memory.
A popular hypothesis dating back to the time of Retzius in the late 19th century asserts that mechanical buckling forces due to the expanding brain tissue cause the cortical surface to fold.
The human cranium continues to grow substantially along with the brain after birth until the cranial plates finally fuse after several years.
[6] The only observed role that the cranium may play in gyrification is in flattening of gyri as the brain matures after the cranial plates fuse.
[13] One study showed that gyrification can be experimentally induced in the embryonic mouse, but at early stages in the absence of axonal connections.
Purely isotropic growth suggests that the grey (outer shell) and white matter (inner core) layers each grow at separate rates, that are uniform in all dimensions.
This level is called a critical point, at which, the models prefer to release potential energy by destabilizing and forming creases to become more stable.
[25][26] These cells rapidly proliferate through self-renewal at early developmental stages, expanding the progenitor pool and increasing cortical surface area.
[27] Cortical neurogenesis begins to deplete the pool of progenitor cells, subject to the influences of many genetic cues such as fibroblast growth factors (FGF)s and Notch.
Both classic RGCs and the recently described bRGCs represent guiding cues that lead newborn neurons to their destination in the cortex.
It has been shown that lissencephalic species possess many of the molecular cues needed to achieve gyrencephaly, but a large variety of genes are involved in the regulation of the neural progenitor proliferation and neurogenic processes that underlie gyrification.
It is hypothesized that spatiotemporal differences in these molecular pathways, including FGF, Shh, and Trnp1 and likely many others, determine the timing and extent of gyrification in various species.
[39][40] A wide array of genes when mutated have been shown to cause Polymicrogyria in humans, ranging from mTORopathies (e.g. AKT3) to channelopathies (sodium channels, "SCN3A").
[41] Patients with autism have overall higher levels of cortical gyrification,[42] but only in the temporal, parietal, and occipital lobes, as well as part of the cingulate cortex.
[43] The higher levels of gyrification are found to relate to greater local connectivity in autistic brains, suggesting hyperconnectivity.
[45] These areas relate to working memory, emotional processing, language, and eye gaze,[46] and their difference in location and level of gyrification when compared to a non-autistic human brain could explain some altered behaviors in autistic patients.