Bicarbonate
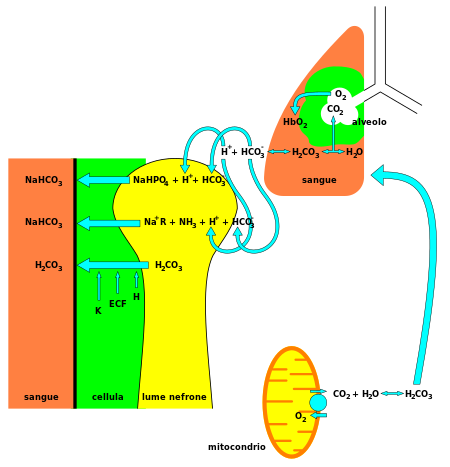
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate[2]) is an intermediate form in the deprotonation of carbonic acid.
The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties.
[citation needed] With carbonic acid as the central intermediate species, bicarbonate – in conjunction with water, hydrogen ions, and carbon dioxide – forms this buffering system, which is maintained at the volatile equilibrium[3] required to provide prompt resistance to pH changes in both the acidic and basic directions.
This is especially important for protecting tissues of the central nervous system, where pH changes too far outside of the normal range in either direction could prove disastrous (see acidosis or alkalosis).
It is released from the pancreas in response to the hormone secretin to neutralize the acidic chyme entering the duodenum from the stomach.
In darkness, when no photosynthesis occurs, respiration processes release carbon dioxide, and no new bicarbonate ions are produced, resulting in a rapid fall in pH.