Human papillomavirus infection
[2] These lesions, depending on the site affected, increase the risk of cancer of the cervix, vulva, vagina, penis, anus, mouth, tonsils, or throat.
[1] These types are typically spread by sustained direct skin-to-skin contact, with vaginal and anal sex being the most common methods.
[14] HPV is not killed by common hand sanitizers and disinfectants, increasing the possibility of the virus being transferred via non-living infectious agents called fomites.
[31] All HPVs are believed to be capable of establishing long-term "latent" infections in small numbers of stem cells present in the skin.
[citation needed] The great majority of genital HPV infections never cause any overt symptoms and are cleared by the immune system in a matter of months.
[34] A large increase in the incidence of genital HPV infection occurs at the age when individuals begin to engage in sexual activity.
[1] Most HPV infections of the cervix are cleared rapidly by the immune system and do not progress to cervical cancer (see below the Clearance subsection in Virology).
[1] Genital HPV is spread by sustained direct skin-to-skin contact, with vaginal, anal, and oral sex being the most common methods.
[88][89] HPV is difficult to remove via standard hospital disinfection techniques and may be transmitted in a healthcare setting on re-usable gynecological equipment, such as vaginal ultrasound transducers.
[citation needed] Partridge reports men's fingertips became positive for high-risk HPV at more than half the rate (26% per two years) as their genitals (48%).
[96][101] Though it has traditionally been assumed that HPV is not transmissible via blood – as it is thought to only infect cutaneous and mucosal tissues – recent studies have called this notion into question.
Surgeons, including urologists and/or anyone in the room, are subject to HPV infection by inhalation of noxious viral particles during electrocautery or laser ablation of a condyloma (wart).
It is believed that involved antibodies play a major neutralizing role while the virions still reside on the basement membrane and cell surfaces.
HPV can survive for many months and at low temperatures without a host; therefore, an individual with plantar warts can spread the virus by walking barefoot.
A sophisticated transcriptional cascade then occurs as the host keratinocyte begins to divide and become increasingly differentiated in the upper layers of the epithelium.
[115] A bioinformatics tool named HPV16-Genotyper performs i) HPV16 lineage genotyping, ii) detects potential recombination events, iii) identifies, within the submitted sequences, mutations/SNPs that have been reported (in literature) to increase the risk for cancer.
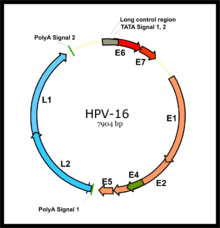
E7 competes for retinoblastoma protein (pRb) binding, freeing the transcription factor E2F to transactivate its targets, thus pushing the cell cycle forward.
In the upper layers of the host epithelium, the late genes L1 and L2 are transcribed/translated and serve as structural proteins that encapsidate the amplified viral genomes.
Once the genome is encapsidated, the capsid appears to undergo a redox-dependent assembly/maturation event, which is tied to a natural redox gradient that spans both suprabasal and cornified epithelial tissue layers.
Others believe that reducing HPV infection in more men and women, even when it has no symptoms, is important (herd immunity) to prevent more cancers rather than just treating them.
[136] The aforementioned Qiagen/Digene kit was successfully used off-label to test the penis, scrotum, and anus[140] of men in long-term relationships with women who were positive for high-risk HPV.
[140][needs update] Similar studies have been conducted on women using cytobrushes - an endocervical brush for sampling the cervix in females - and custom analysis.
[96][101][146][needs update] Other studies analyzed urine, semen, and blood and found varying amounts of HPV,[136] but there is not a publicly available test for those yet.
Five percent acetic acid (vinegar) is used to identify both warts and squamous intraepithelial neoplasia (SIL) lesions with limited success[citation needed] by causing abnormal tissue to appear white, but most doctors have found this technique helpful only in moist areas, such as the female genital tract.
[177] According to the Centers for Disease Control and Prevention, the body's immune system clears HPV naturally within two years for 90% of cases (see Clearance subsection in Virology for more detail).
In addition to the normal methods of phone calls and mail, text messaging and email can improve the number of people who return for care.
The higher rates of HPV in Sub-Saharan Africa, for example, may be related to high exposure to human immunodeficiency virus (HIV) in the region.
[202] Urbanization, the number of sex partners, and PAP history appear as risk factors for HPV infection in Han, but not in Mongolian women.
[citation needed] In 1972, the association of the human papillomaviruses with skin cancer in epidermodysplasia verruciformis was proposed by Stefania Jabłońska in Poland.
In 1976 Harald zur Hausen published the hypothesis that human papillomavirus plays an important role in the cause of cervical cancer.