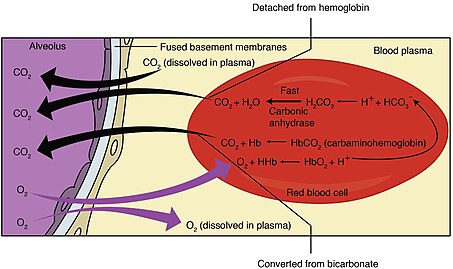
Haldane effect
The Haldane effect is a property of hemoglobin first described by John Scott Haldane, within which oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin, increasing the removal of carbon dioxide.
Amino groups are available for binding at the N-terminals and at side-chains of arginine and lysine residues in hemoglobin.
These dynamics explain the relative difference in hemoglobin's affinity for carbon dioxide depending on oxygen levels known as the Haldane effect.
[1] In red blood cells, the enzyme carbonic anhydrase catalyzes the conversion of dissolved carbon dioxide to carbonic acid, which rapidly dissociates to bicarbonate and a free proton: By Le Chatelier's principle, anything that stabilizes the proton produced will cause the reaction to shift to the right, thus the enhanced affinity of deoxyhemoglobin for protons enhances synthesis of bicarbonate and accordingly increases capacity of deoxygenated blood for carbon dioxide.
In the oxygen-rich capillaries of the lung, this property causes the displacement of carbon dioxide to plasma as low-oxygen blood enters the alveolus and is vital for alveolar gas exchange.