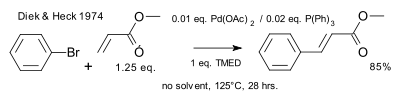
Heck reaction
Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction.
[2][3][4][5] The original reaction by Tsutomu Mizoroki (1971) describes the coupling between iodobenzene and styrene in methanol to form stilbene at 120 °C (autoclave) with potassium acetate base and palladium chloride catalysis.
Step C involves a beta-hydride elimination (here the arrows are showing the opposite) with the formation of a new palladium - alkene π complex (5).
The cycle also extends to the other group 10 element nickel for example in the Negishi coupling between aryl halides and organozinc compounds.
The naproxen synthesis includes a coupling between a brominated naphthalene compound with ethylene:[15] In the presence of an ionic liquid a Heck reaction proceeds in absence of a phosphorus ligand.
In one modification palladium acetate and the ionic liquid (bmim)PF6 are immobilized inside the cavities of reversed-phase silica gel.
In the Heck oxyarylation modification the palladium substituent in the syn-addition intermediate is displaced by a hydroxyl group and the reaction product contains a dihydrofuran ring.
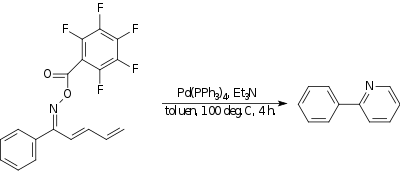
In one example,[18] an oxime with a strongly electron withdrawing group reacts intramolecularly with the end of a diene to form a pyridine compound.