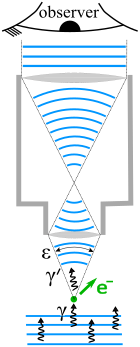
Heisenberg's microscope
The concept was criticized[clarification needed] by Heisenberg's mentor Niels Bohr, and theoretical and experimental developments have suggested that Heisenberg's intuitive explanation[clarification needed] of his mathematical result might be misleading.
The formal mathematical result remains valid, however, and the original intuitive argument has also been vindicated mathematically when the notion of disturbance[clarification needed] is expanded to be independent of any specific state.
Let the cone of light rays leaving the microscope lens and focusing on the electron make an angle
Then, according to the laws of classical optics, the microscope can only resolve the position of the electron up to an accuracy of[5]: 21 [6] An observer perceives an image of the particle because the light rays strike the particle and bounce back through the microscope to the observer's eye.
We know from experimental evidence that when a photon strikes an electron, the latter has a Compton recoil with momentum proportional to
However, the extent of "recoil cannot be exactly known, since the direction of the scattered photon is undetermined within the bundle of rays entering the microscope.
[citation needed] Although the thought experiment was formulated as an introduction to Heisenberg's uncertainty principle, one of the pillars of modern physics, it attacks the very premises under which it was constructed, thereby contributing to the development of an area of physics—namely, quantum mechanics—that redefined the terms under which the original thought experiment was conceived.
In other words, the "position" of an electron can only be stated in terms of a probability distribution, as can predictions of where it may move.