Helicase
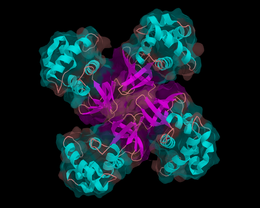
Helicases are motor proteins that move directionally along a nucleotidic backbone, separating two hybridized nucleic acid strands (hence helic- + -ase), using energy from ATP hydrolysis.
Some specialized helicases are also involved in sensing of viral nucleic acids during infection and fulfill an immunological function.
Helicase is an essential component of cellular mechanisms that ensures accurate DNA replication and maintenance of genetic information.
RecG replaces the single-strand binding protein (SSB), which regulates the helicase-fork loading sites during fork regression.
Thermal sliding and DNA duplex binding are possibly supported by the wedge domain of RecG's association with the SSB linker.
Helicases are often used to separate strands of a DNA double helix or a self-annealed RNA molecule using the energy from ATP hydrolysis, a process characterized by the breaking of hydrogen bonds between annealed nucleotide bases.
[3] Helicases move incrementally along one nucleic acid strand of the duplex with a directionality and processivity specific to each particular enzyme.
[6] Helicases may process much faster in vivo than in vitro due to the presence of accessory proteins that aid in the destabilization of the fork junction.
[7][5][8][9] The activation barrier is a result of various factors, and can be defined by where Factors that contribute to the height of the activation barrier include: specific nucleic acid sequence of the molecule involved, the number of base pairs involved, tension present on the replication fork, and destabilization forces.
) (translocation along the single-strand nucleic acid, ssNA), due to its reliance on the transient unraveling of the base pairs at the replication fork to determine its rate of unwinding.
In such models, the passive helicases are conceptualized as Brownian ratchets, driven by thermal fluctuations and subsequent anisotropic gradients across the DNA lattice.
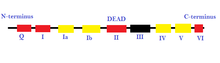
[16] Below is a history of helicase discovery: The common function of helicases accounts for the fact that they display a certain degree of amino acid sequence homology; they all possess sequence motifs located in the interior of their primary structure, involved in ATP binding, ATP hydrolysis and translocation along the nucleic acid substrate.
[35] XPD (Xeroderma pigmentosum factor D, also known as protein ERCC2) is a 5'-3', Superfamily II, ATP-dependent helicase containing iron-sulphur cluster domains.
[26][37] Inherited point mutations in XPD helicase have been shown to be associated with accelerated aging disorders such as Cockayne syndrome (CS) and trichothiodystrophy (TTD).
[38] The XPD helicase mutation has also been implicated in xeroderma pigmentosum (XP), a disorder characterized by sensitivity to UV light and resulting in a several 1000-fold increase in the development of skin cancer.
[38][39][40][41][42] A mutation in the XPD helicase that helps form this complex and contributes to its function causes the sensitivity to sunlight seen in all three diseases, as well as the increased risk of cancer seen in XP and premature aging seen in trichothiodystrophy and Cockayne syndrome.
[43][44] Deficiencies and/or mutations in RecQ family helicases display aberrant genetic recombination and/or DNA replication, which leads to chromosomal instability and an overall decreased ability to proliferate.
[44][46] Cells of Bloom syndrome patients show a high frequency of reciprocal exchange between sister chromatids (SCEs) and excessive chromosomal damage.
RecQ is a family of DNA helicase enzymes that are found in various organisms including bacteria, archaea, and eukaryotes (like humans).
For instance, mutations in the BLM gene cause Bloom syndrome, which is characterized by increased cancer risk and other health issues.
[50] Mutations in the WRN gene lead to Werner syndrome, a condition characterized by premature aging and an increased risk of age-related diseases.
[52] The RecQ helicase family member, RECQ1, is connected to a small number of uncommon genetic cancer disorders in individuals.
Chemical manipulation of their molecular processes can change the rate at which cancer cells divide, as well as, the efficiency of transactions and cellular homeostasis.
[54] These helicases, through their ability to unwind D-loop intermediates, promote NCO recombination by the process of synthesis-dependent strand annealing.
It was suggested that COs are restricted because of the long term costs of CO recombination, that is, the breaking up of favourable genetic combinations of alleles built up by past natural selection.
[56] Some neurological disorders associated with defective RNA helicases are: amyotrophic lateral sclerosis, spinal muscular atrophy, spinocerebellar ataxia type-2, Alzheimer disease, and lethal congenital contracture syndrome.
Other methods were later developed that incorporated some, if not all of the following: high-throughput mechanics, the use of non-radioactive nucleotide labeling, faster reaction time/less time consumption, real-time monitoring of helicase activity (using kinetic measurement instead of endpoint/single point analysis).
[16] With the use of specialized mathematical equations, some of these assays can be utilized to determine how many base paired nucleotides a helicase can break per hydrolysis of 1 ATP molecule.
The basis of this assay is the "quenching" or repressing of the lanthanide chelate signal by the organic quencher molecule when the two are in close proximity – as they would be when the DNA duplex is in its native state.
This loss in proximity negates the quenchers ability to repress the lanthanide signal, causing a detectable increase in fluorescence that is representative of the amount of unwound DNA and can be used as a quantifiable measurement of helicase activity.