Hess's law
The law states that the total enthalpy change during the complete course of a chemical reaction is independent of the sequence of steps taken.
According to the first law of thermodynamics, the enthalpy change in a system due to a reaction at constant pressure is equal to the heat absorbed (or the negative of the heat released), which can be determined by calorimetry for many reactions.
Hess's law can be used to determine the overall energy required for a chemical reaction that can be divided into synthetic steps that are individually easier to characterize.
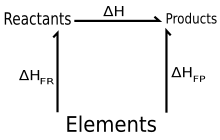
Hess's law states that the change of enthalpy in a chemical reaction is the same regardless of whether the reaction takes place in one step or several steps, provided the initial and final states of the reactants and products are the same.
Enthalpy is an extensive property, meaning that its value is proportional to the system size.
Hess's law allows the enthalpy change (ΔH) for a reaction to be calculated even when it cannot be measured directly.
This is accomplished by performing basic algebraic operations based on the chemical equations of reactions using previously determined values for the enthalpies of formation.
The concepts of Hess's law can be expanded to include changes in entropy and in Gibbs free energy, since these are also state functions.
The Bordwell thermodynamic cycle is an example of such an extension that takes advantage of easily measured equilibria and redox potentials to determine experimentally inaccessible Gibbs free energy values.