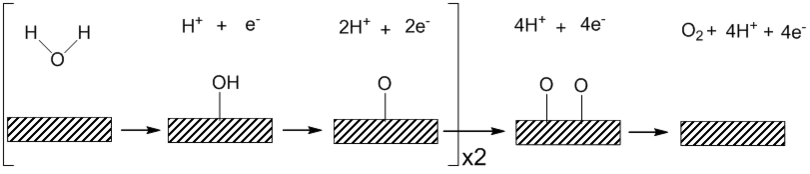
Heterogeneous water oxidation
Figure 1 shows the standard potentials at pH 0 (strongly acidic) as referenced to the normal hydrogen electrode (NHE).
Systems always suffer from an overpotential that arise from activation barriers, concentration effects and voltage drops due to resistance.
Under acidic conditions water binds to the surface with the irreversible removal of one electron and one proton to form a platinum hydroxide.
[5] The shift in mechanism between the pH extremes has been attributed to the kinetic facility of oxidizing hydroxide ion relative to water.
[7] OER has been studied on a variety of materials including: Preparation of the surface and electrolysis conditions have a large effect on reactivity (defects, steps, kinks, low coordinate sites) therefore it is difficult to predict an OER material's properties by its bulk structure.
It has been studied since the early 1970s as a water oxidation catalyst with one of the lowest reported overpotentials for OER at the time.
Generally these spinels are ofter coated over the carbon materials and reduced further to create oxygen vacancy in their lattice to enhance the water oxidation capabilities.