Nernst equation
The cell potential E associated with the electrochemical reaction is defined as the decrease in Gibbs free energy per coulomb of charge transferred, which leads to the relationship
Similarly to equilibrium constants, activities are always measured with respect to the standard state (1 mol/L for solutes, 1 atm for gases, and T = 298.15 K, i.e., 25 °C or 77 °F).
When wishing to use simple concentrations in place of activities, but that the activity coefficients are far from unity and can no longer be neglected and are unknown or too difficult to determine, it can be convenient to introduce the notion of the "so-called" standard formal reduction potential (
is the reduction potential that applies to a half reaction under a set of specified conditions such as, e.g., pH, ionic strength, or the concentration of complexing agents.
[3] Several definitions of the formal reduction potential can be found in the literature, depending on the pursued objective and the experimental constraints imposed by the studied system.
The formal reduction potential makes possible to more simply work with molar (mol/L, M) or molal (mol/kg H2O, m) concentrations in place of activities.
[8]In this case, as for the standard reduction potentials, the concentrations of dissolved species remain equal to one molar (M) or one molal (m), and so are said to be one formal (F).
cannot be referred as an immutable standard potential but needs to be systematically determined for each specific set of experimental conditions.
[7] Formal reduction potentials are applied to simplify calculations of a considered system under given conditions and measurements interpretation.
) allow to more easily estimate if a redox reaction supposed to occur in a metabolic process or to fuel microbial activity under some conditions is feasible or not.
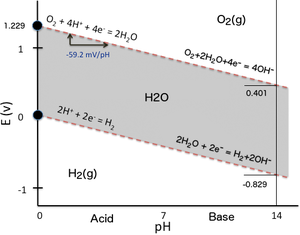
and pH of a solution are related by the Nernst equation as commonly represented by a Pourbaix diagram (
is the standard Gibbs free energy change, z is the number of electrons involved, and F is the Faraday's constant.
The main factor affecting the formal reduction potentials in biochemical or biological processes is most often the pH.
To determine approximate values of formal reduction potentials, neglecting in a first approach changes in activity coefficients due to ionic strength, the Nernst equation has to be applied taking care to first express the relationship as a function of pH.
When working at the frontier between inorganic and biological processes (e.g., when comparing abiotic and biotic processes in geochemistry when microbial activity could also be at work in the system), care must be taken not to inadvertently directly mix standard reduction potentials versus SHE (pH = 0) with formal reduction potentials (pH = 7).
To illustrate the dependency of the reduction potential on pH, one can simply consider the two oxido-reduction equilibria determining the water stability domain in a Pourbaix diagram (Eh–pH plot).
In the Eh–pH plot here beside (the simplest possible version of a Pourbaix diagram), the water stability domain (grey surface) is delimited in term of redox potential by two inclined red dashed lines: When solving the Nernst equation for each corresponding reduction reaction (need to revert the water oxidation reaction producing oxygen), both equations have a similar form because the number of protons and the number of electrons involved within a reaction are the same and their ratio is one (2 H+/2 e− for H2 and 4 H+/4 e− with O2 respectively), so it simplifies when solving the Nernst equation expressed as a function of pH.
is the same for both reduction reactions because they share the same linear relationship as a function of pH and the slopes of their lines are the same.
Beside important redox reactions in biochemistry and microbiology, the Nernst equation is also used in physiology for calculating the electric potential of a cell membrane with respect to one type of ion.
As seen above, the magnitude of the Nernst potential is determined by the ratio of the concentrations of that specific ion on the two sides of the membrane.
Fundamental statistical proof of the mentioned linearity goes beyond the scope of this section, but to see this is true it is simpler to consider usual isothermal process for an ideal gas where the change of entropy ΔS = nR ln(V2/V1) takes place.
In this sense there is no difference in statistical properties of ideal gas atoms compared with the dissolved species of a solution with activity coefficients equaling one: particles freely "hang around" filling the provided volume), which is inversely proportional to the concentration c, so we can also write the entropy as
, which includes the activity coefficients of the dissolved species under given experimental conditions (T, P, ionic strength, pH, and complexing agents) and is the potential that is actually measured in an electrochemical cell.
In dilute solutions, the Nernst equation can be expressed directly in the terms of concentrations (since activity coefficients are close to unity).
This complicates the use of the Nernst equation, since estimation of non-ideal activities of ions generally requires experimental measurements.
The activity of ions at the electrode surface changes when there is current flow, and there are additional overpotential and resistive loss terms which contribute to the measured potential.
This is physically meaningless because, under such conditions, the exchange current density becomes very low, and there may be no thermodynamic equilibrium necessary for Nernst equation to hold.
Other effects tend to take control of the electrochemical behavior of the system, like the involvement of the solvated electron in electricity transfer and electrode equilibria, as analyzed by Alexander Frumkin and B. Damaskin,[13] Sergio Trasatti, etc.
Fleischmann and Pons, claiming that cold fusion could exist, calculated that a palladium cathode immersed in a heavy water electrolysis cell could achieve up to 1027 atmospheres of pressure inside the crystal lattice of the metal of the cathode, enough pressure to cause spontaneous nuclear fusion.
The American physicist John R. Huizenga claimed their original calculation was affected by a misinterpretation of the Nernst equation.