Heterogeneous catalysis
Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures (e.g., oil and water), or anywhere an interface is present.
The Lennard-Jones model provides a basic framework for predicting molecular interactions as a function of atomic separation.
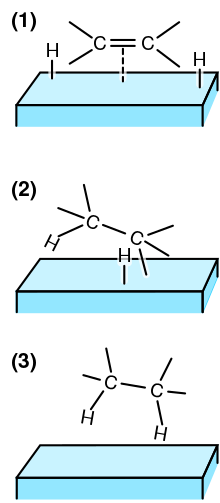
[7] When a molecule approaches close enough to surface atoms such that their electron clouds overlap, chemisorption can occur.
[2] Two cases of chemisorption are: Most metal surface reactions occur by chain propagation in which catalytic intermediates are cyclically produced and consumed.
[10] Porous materials are cost effective due to their high surface area-to-mass ratio and enhanced catalytic activity.
In many cases, a solid catalyst is dispersed on a supporting material to increase surface area (spread the number of active sites) and provide stability.
Most catalyst supports are porous (frequently carbon, silica, zeolite, or alumina-based)[4] and chosen for their high surface area-to-mass ratio.
Often, substances are intentionally added to the reaction feed or on the catalyst to influence catalytic activity, selectivity, and/or stability.
For example, alumina (Al2O3) is added during ammonia synthesis to providing greater stability by slowing sintering processes on the Fe-catalyst.
[11] Sabatier principle states that the surface-adsorbates interaction has to be an optimal amount: not too weak to be inert toward the reactants and not too strong to poison the surface and avoid desorption of the products.
[15] Applying scaling relations to the catalyst design problems greatly reduces the space dimensionality (sometimes to as small as 1 or 2).
[16] One can also use micro-kinetic modeling based on such scaling relations to take into account the kinetics associated with adsorption, reaction and desorption of molecules under specific pressure or temperature conditions.
[17] Such modeling then leads to well-known volcano-plots at which the optimum qualitatively described by the Sabatier principle is referred to as the "top of the volcano".
[19] The correlations which are manifested in the scaling relations confine the catalyst design space, preventing one from reaching the "top of the volcano".
Poisons chemisorb to catalyst surface and reduce the number of available active sites for reactant molecules to bind to.
[22] Common poisons include Group V, VI, and VII elements (e.g. S, O, P, Cl), some toxic metals (e.g. As, Pb), and adsorbing species with multiple bonds (e.g. CO, unsaturated hydrocarbons).
[23] The presence of poisons and promoters can alter the activation energy of the rate-limiting step and affect a catalyst's selectivity for the formation of certain products.