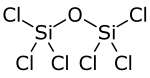
Hexachlorodisiloxane
Hexachlorodisiloxane is a chemical compound composed of chlorine, silicon, and oxygen.
Structurally, it is the symmetrical ether of two trichlorosilyl groups, and can be synthesized via high-temperature oxidation of silicon tetrachloride:
SiCl
SiCl
At room temperature, it is a colorless liquid that hydrolyzes upon exposure to water to give silicon dioxide and hydrochloric acid:
SiCl
Intense heat evinces a similar decomposition:
Reaction with antimony trifluoride gives the analogous hexafluorodisiloxane.
This inorganic compound–related article is a stub.
You can help Wikipedia by expanding it.