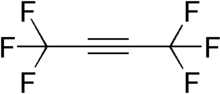Hexafluoro-2-butyne
HFB is a particularly electrophilic acetylene derivative, and hence a potent dienophile for Diels–Alder reactions.
[2][3] HFB is prepared by the action of sulfur tetrafluoride on acetylenedicarboxylic acid or by the reaction of potassium fluoride (KF) with hexachlorobutadiene.
In the presence of the strong Lewis acid aluminium chlorofluoride, hexafluorobutadiene isomerizes to HFB:[4] HFB reacts with sulfur to give 3,4-bis(trifluoromethyl)-1,2-dithiete.
This derivative can be reduced to the 7 electron neutral radical.
This particular 1,3,5-dithiazole is also rare example of a radical that can be obtained as solid, liquid, and gaseous states.