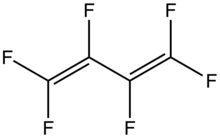
Hexafluorobutadiene
Hexafluorobutadiene is an organofluorine compound with the formula (CF2=CF)2.
A colorless gas, it has attracted attention as an etchant in microelectronics.
It can be prepared by coupling of fluorinated C2 precursors.
Addition of iodine monochloride to chlorotrifluoroethylene gives iododichlorotrifluoroethane that can be coupled in the presence of mercury to give 1,2,3,4-tetrchlorohexafluorobutane: Zn-induced dechlorination of this tetrachloride gives the desired perfluorinated diene:[1] The diene can be rehalogenated, e.g. with bromine upon UV irradiation:[1] Hexafluorobutadiene dimerizes via a [2+2] process at 150 °C to give perfluorinated divinylcyclobutanes.
[2] In the presence of the strong Lewis acid aluminium chlorofluoride, hexafluorobutadiene isomerizes to hexafluoro-2-butyne:[3]