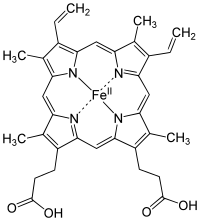
Heme
[2] Heme plays a critical role in multiple different redox reactions in mammals, due to its ability to carry the oxygen molecule.
[6] Among the metalloporphyrins deployed by metalloproteins as prosthetic groups, heme is one of the most widely used[7] and defines a family of proteins known as hemoproteins.
During the detection of diatomic gases, the binding of the gas ligand to the heme iron induces conformational changes in the surrounding protein.
It has been speculated that the original evolutionary function of hemoproteins was electron transfer in primitive sulfur-based photosynthesis pathways in ancestral cyanobacteria-like organisms before the appearance of molecular oxygen.
[12] For example, the ability of hemoglobin to effectively deliver oxygen to tissues is due to specific amino acid residues located near the heme molecule.
[13] Hemoglobin reversibly binds to oxygen in the lungs when the pH is high, and the carbon dioxide concentration is low.
When the situation is reversed (low pH and high carbon dioxide concentrations), hemoglobin will release oxygen into the tissues.
This phenomenon, which states that hemoglobin's oxygen binding affinity is inversely proportional to both acidity and concentration of carbon dioxide, is known as the Bohr effect.
[14] The molecular mechanism behind this effect is the steric organization of the globin chain; a histidine residue, located adjacent to the heme group, becomes positively charged under acidic conditions (which are caused by dissolved CO2 in working muscles, etc.
The rate-limiting enzyme responsible for this reaction, ALA synthase, is negatively regulated by glucose and heme concentration.
This mechanism is of therapeutic importance: infusion of heme arginate or hematin and glucose can abort attacks of acute intermittent porphyria in patients with an inborn error of metabolism of this process, by reducing transcription of ALA synthase.
However, due to its toxic properties, proteins such as emopexin (Hx) are required to help maintain physiological stores of iron in order for them to be used in synthesis.
The DNA for leghemoglobin production was extracted from the soybean root nodules and expressed in yeast cells to overproduce heme for use in the meatless burgers.
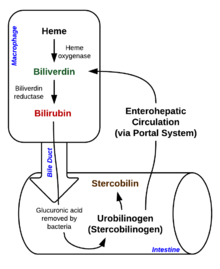
[36] NADPH is used as the reducing agent, molecular oxygen enters the reaction, carbon monoxide (CO) is produced and the iron is released from the molecule as the ferrous ion (Fe2+).
[49] There is an association between high intake of heme iron sourced from meat and increased risk of colorectal cancer.