Hydrogen-bonded organic framework
Hydrogen-bonded organic frameworks (HOFs) are a class of porous polymers formed by hydrogen bonds among molecular monomer units to afford porosity and structural flexibility.
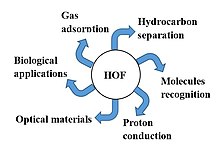
[5][6] An important consequence[editorializing] of the natural porous architecture of hydrogen-bonded organic frameworks is to realize the adsorption of guest molecules.
In 2011, Chen reported a porous organic framework with hydrogen bonding as binding force and demonstrated its porosity by gas adsorption for the first time.
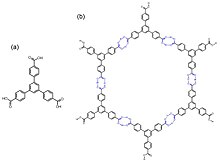
The hydrogen bonding pairs generally include 2,4-diaminotriazine, carboxylic acid, amide, imide, imidazole, imidazolone and resorcinol, etc.
Also, by using backbones with similar geometry and same connection pattern to generate the monomers and HOFs, the isoreticular expansion of the frameworks becomes a reliable method to expand the pore size effectively.
[22][23] As mentioned, for the sake of constructing porous and stable HOFs, multiple aspects should be considered simultaneously, such as the rigidity of the backbones, the orientation and binding strength of the hydrogen pairs, and other intermolecular interactions for orderly stacking.
Therefore, the design of HOF monomers should focus on their H-bonds orientations and structural rigidity, and consequent framework stability and porosity.
Nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (HR-MS) are generally used for characterizing the synthesis of monomers.
Because of the molecular rigidity and stereo construction, 1D channels were formed through the frameworks and the surface area was largely enhanced, to the extent of 2796 m2/g as shown by BET.
The CO2 uptake capacity of the HOF could reach 117.1 cm3/g at 273 K.[citation needed] The hydrogen-bonded organic framework used for C2H2/C2H4 separation was reported by Chen and coworkers.
[33][34] As kinds of metal-free porous materials, hydrogen-bonded organic frameworks are also ideal platform for drug delivery and disease treatment.
[35] Meanwhile, with proper monomer selection and reasonable arrangement, Cao reported a robust HOF which could effectively encapsulate a cancer drug Doxorubicin and yield singlet oxygen by embedded photoactive pyrene moiety in order to realize dual functions of drug release and photodynamic therapy for cancer remedy.