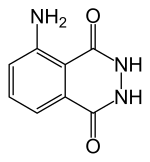
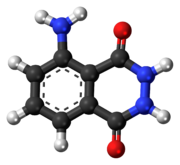
Luminol
Forensic investigators use luminol to detect trace amounts of blood at crime scenes, as it reacts with the iron in hemoglobin.
Biologists use it in cellular assays to detect copper, iron, and cyanides as well as specific proteins via western blotting.
[8] Luminol is first deprotonated in basic conditions then oxidized to the anionic radical, which in turn has two paths available to give the key intermediate α-hydroxy- peroxide.
This highly instable molecule relaxes to the ground state, thereby emitting light of around 425 nm wavelength (purple-blue), thus chemiluminescence.
In 1928, German chemist H. O. Albrecht found that blood, among other substances, enhanced the luminescence of luminol in an alkaline solution of hydrogen peroxide.
[11] In 1937, German forensic scientist Walter Specht made extensive studies of luminol's application to the detection of blood at crime scenes.
Luminol's use in a crime scene investigation is somewhat hampered by the fact that it reacts to iron- and copper-containing compounds,[15] bleaches, horseradish, fecal matter, and cigarette smoke residue.