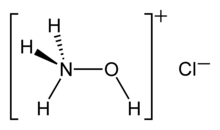
Hydroxylammonium chloride
During the acetyl bromide method of extracting lignin from lignocellulosic biomass, hydroxylammonium chloride can be used to remove bromine and polybromide from the solution.
In surface treatments, it is used in the preparation of anti-skinning agents, corrosion inhibitors, and cleaner additives.
It is also used as a fixative for textile dyes, auxiliary in some dyeing processes, as a metal extraction and flotation aid, as an antioxidant in fatty acids and soaps, and as a color stabilizer and emulsion additive in color films.
It is also used in analytic chemistry in the analysis of iron in the water combined with α,α-dipyridyl.
The hydroxylammonium chloride transforms all the iron in Fe2+, that then forms a coordination complex with the dipyridyl.