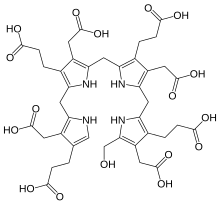
Hydroxymethylbilane
The compound is a substituted bilane, a chain of four pyrrole rings interconnected by methylene bridges −CH2−.
The chain starts with a hydroxymethyl group −CH2−OH and ends with an hydrogen, in place of the respective methylene bridges.
[1] HMB is generated from four molecules of porphobilinogen by the enzyme porphobilinogen deaminase:[2] The enzyme uroporphyrinogen III synthase closes the chain to form uroporphyrinogen III:[2]
Uroporphyrinogen III is a porphyrinogen, which is a class of compounds with the hexahydroporphine macrocycle.
In the absence of the enzyme, the compound undergoes spontaneous cyclization and becomes uroporphyrinogen I.