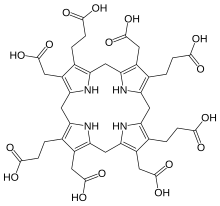
Uroporphyrinogen III
Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme.
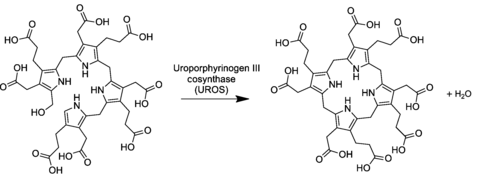
In the general porphyrin biosynthesis pathway, uroporphyrinogen III is derived from the linear tetrapyrrole preuroporphyrinogen (a substituted hydroxymethylbilane) by the action of the enzyme uroporphyrinogen-III cosynthase.
The conversion entails a reversal of the last pyrrole unit (thus swapping the acetic and propionic acid groups) and a condensation reaction that closes the macrocycle by eliminating the final hydroxyl −OH with a hydrogen atom of the first ring.
If uroporphyrinogen-III synthase is not present or inactive, the hydroxymethylbilane will spontaneously cyclise into the structural isomer uroporphyrinogen I, which differs from the III isomer in that the acetic acid ("A") and propionic acid ("P") groups are arranged in a rotationally symmetric order, AP-AP-AP-AP.
In this case, the next step produced coproporphyrinogen I, which accumulates — leading to the pathological condition congenital erythropoietic porphyria[3]