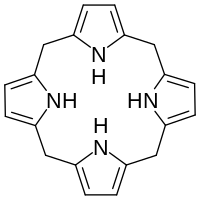
Hexahydroporphine
The molecule consists of four pyrrole rings connected by methylene bridges −CH2− into a larger (non-aromatic) macrocycle ring, which makes it one of the simplest tetrapyrroles, and the simplest "true" one.
Hexahydroporphine does not occur in nature, but is the core of porphyrinogens such as uroporphyrinogen III (UROGEN), which are precursors of many porphyrins — derivatives of porphine of great biological importance.
The six hydrogens of that core are removed at a later metabolic stage by the enzyme protoporphyrinogen oxidase.
The compound is a colorless solid, soluble in dichloromethane, acetone, and diethyl ether.
[1] Derivatives of hexahydroporphine, with various groups attached to the pyrrole or methylene bridges, occur in nature and have been studied for a long time.