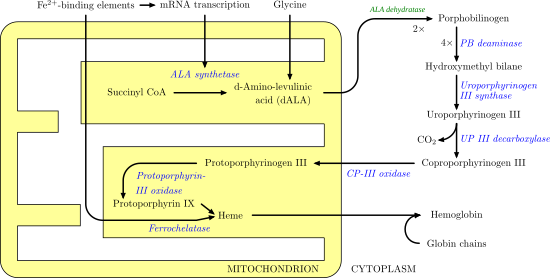
Porphobilinogen deaminase
[5][6] Domains 1 and 2 are structurally very similar: each consisting of five beta-sheets and three alpha helices in humans.
[8] Several positively charged arginine residues, positioned to face the active site from domains 1 and 2, have been shown to stabilize the carboxylate functionalities on the incoming porphobilinogen as well as the growing pyrrole chain.
The first step is believed to involve an E1 elimination of ammonia from porphobilinogen, generating a carbocation intermediate (1).
[10] This intermediate is then attacked by the dipyrrole cofactor of porphobilinogen deaminase, which after losing a proton yields a trimer covalently bound to the enzyme (2).
[11][12] The most well-known health issue involving porphobilinogen deaminase is acute intermittent porphyria, an autosomal dominant genetic disorder where insufficient hydroxymethylbilane is produced, leading to a build-up of porphobilinogen in the cytoplasm.