Ideal chain
In other cases, a monomer is simply a segment of the polymer that can be modeled as behaving as a discrete, freely jointed unit.
For example, chromatin is modeled as a polymer in which each monomer is a segment approximately 14–46 kbp in length.
In this very simple approach where no interactions between mers are considered, the energy of the polymer is taken to be independent of its shape, which means that at thermodynamic equilibrium, all of its shape configurations are equally likely to occur as the polymer fluctuates in time, according to the Maxwell–Boltzmann distribution.
Most of the expressions given in this article assume that the number of mers N is large, so that the central limit theorem applies.
It is worth noting that the above average end-to-end distance, which in the case of this simple model is also the typical amplitude of the system's fluctuations, becomes negligible compared to the total unfolded length of the polymer
is not as direct as it appears above: from the application of the usual (1D) central limit theorem one can deduce that
Alternatively, this result can also be demonstrated by applying a multidimensional generalization of the central limit theorem, or through symmetry arguments.
While the elementary model described above is totally unadapted to the description of real-world polymers at the microscopic scale, it does show some relevance at the macroscopic scale in the case of a polymer in solution whose monomers form an ideal mix with the solvent (in which case, the interactions between monomer and monomer, solvent molecule and solvent molecule, and between monomer and solvent are identical, and the system's energy can be considered constant, validating the hypotheses of the model).
The relevancy of the model is, however, limited, even at the macroscopic scale, by the fact that it does not consider any excluded volume for monomers (or, to speak in chemical terms, that it neglects steric effects).
Since the N mers are of a rigid, fixed length, the model also does not consider bond stretching, though it can be extended to do so.
As the ideal chain is stretched, its energy remains constant, and its time-average, or internal energy, also remains constant, which means that this force necessarily stems from a purely entropic effect.
This entropic force is very similar to the pressure experienced by the walls of a box containing an ideal gas.
The internal energy of an ideal gas depends only on its temperature, and not on the volume of its containing box, so it is not an energy effect that tends to increase the volume of the box like gas pressure does.
In other words, thermal fluctuations tend to bring a system toward its macroscopic state of maximum entropy.
In its solvent, the ideal chain is constantly subject to shocks from moving solvent molecules, and each of these shocks sends the system from its current microscopic state to another, very similar microscopic state.
Thus, for an ideal chain, maximizing its entropy means reducing the distance between its two free ends.
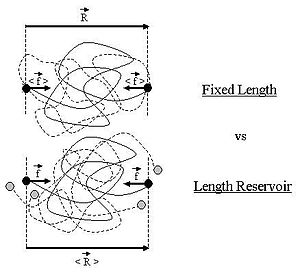
The case of an ideal chain whose two ends are attached to fixed points will be considered in this sub-section.
joining these two points characterizes the macroscopic state (or macro-state) of the ideal chain.
Suppose that, instead of being fixed, the positions of the two ends of the ideal chain are now controlled by an operator.
is defined as the elementary amount of mechanical work transferred by the operator to the ideal chain, and
is defined as the elementary amount of heat transferred by the solvent to the ideal chain.
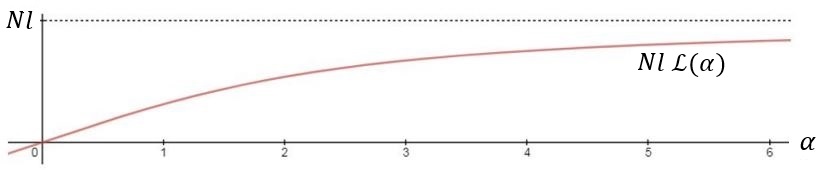
It is also only valid for small end-to-end distances, relative to the overall polymer contour length, where the behavior is like a hookean spring.
Behavior over larger force ranges can be modeled using a canonical ensemble treatment identical to magnetization of paramagnetic spins.
Finally, the model can be extended to even larger force ranges by inclusion of a stretch modulus along the polymer contour length.
That is, by allowing the length of each unit of the chain to respond elastically to the applied force.
For an ideal chain exchanging length with a reservoir, a macro-state of the system is characterized by the vector
The change between an ideal chain of fixed length and an ideal chain in contact with a length reservoir is very much akin to the change between the micro-canonical ensemble and the canonical ensemble (see the Statistical mechanics article about this).
As in the micro-canonical and canonical ensembles, the two descriptions of the ideal chain differ only in the way they treat the system's fluctuations.
The Gibbs free energy is used here because the ensemble of chains corresponds to constant temperature
The average end-to-end distance corresponding to a given force can be obtained as the derivative of the free energy: