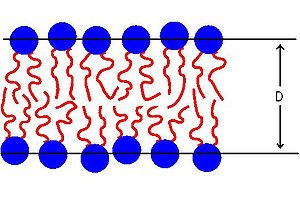
Interbilayer forces in membrane fusion
It is defined as the event where two lipid bilayers approach each other and then merge to form a single continuous structure.
This repulsion was first proposed by Langmuir and was thought to arise due to water molecules that hydrate the bilayers.
[7] Due to their long range nature, they are responsible for rapid coagulation of hydrophobic particles in water and play important roles in various biological phenomena including folding and stabilization of macromolecules such as proteins and fusion of cell membranes.
During fusion, the hydrophobic tails of a small patch of lipids on the cell membrane are exposed to the aqueous phase surrounding them.
This results in very strong hydrophobic attractions (which dominate the repulsive force) between the exposed groups leading to membrane fusion.
[10] The attractive van der Waals forces play a negligible role in membrane fusion.
Thus, fusion is a result of the hydrophobic attractions between internal hydrocarbon chain groups that are exposed to the normally inaccessible aqueous environment.
[7] Interbilayer forces play a key role in mediating membrane fusion, which has extremely important biomedical applications.