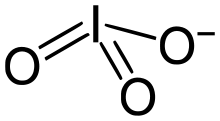
Iodate
It is the most common form of iodine in nature, as it comprises the major iodine-containing ores.
Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate.
[3] Iodate is unusual in that it forms a strong hydrogen bond with its parent acid:[2] The anion H(IO3)−2 is referred to as biiodate.
Minerals containing iodate are found in the caliche deposits of Chile.
[7] Natural waters contain iodine in the form of iodide and iodate, their ratio being dependent on redox conditions and pH.