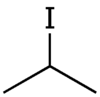
Isopropyl iodide
Isopropyl iodide is the organoiodine compound with the formula (CH3)2CHI.
It is colorless, flammable, and volatile.
Organic iodides are light-sensitive and take on a yellow colour upon storage, owing to the formation of iodine.
Isopropyl iodide is prepared by iodination of isopropyl alcohol using hydrogen iodide or, equivalently, with a mixture of glycerol, iodine, and phosphorus.
[2] An alternative preparation involves the reaction of 2-propyl bromide with an acetone solution of sodium iodide (Finkelstein reaction):[3] This article about an organic halide is a stub.