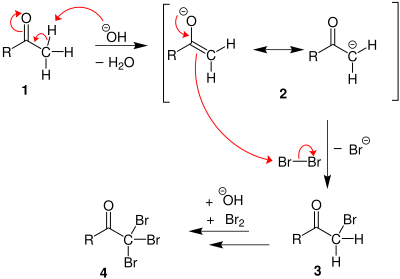
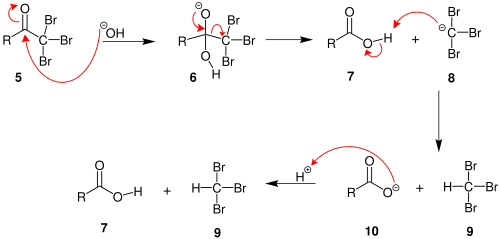
Haloform reaction
The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge).
At least in some cases (chloral hydrate) the reaction may stop and the intermediate product isolated if conditions are acidic and hypohalite is used.
[4] Fluoroform (CHF3) cannot be prepared by this method as it would require the presence of the highly unstable hypofluorite ion.
However ketones with the structure RCOCF3 do cleave upon treatment with base to produce fluoroform; this is equivalent to the second and third steps in the process shown above.
When iodine and sodium hydroxide are used as the reagents a positive reaction gives iodoform, which is a solid at room temperature and tends to precipitate out of solution causing a distinctive cloudiness.
In organic chemistry, this reaction may be used to convert a terminal methyl ketone into the analogous carboxylic acid.