Iron-rich sedimentary rocks
[1] The main iron ores are from the oxide group consisting of hematite, goethite, and magnetite.
Generally, they are from the Phanerozoic which means that they range in age from the present to 540 million years ago.
However, iron formations are mainly Precambrian in age which means that they are 4600 to 590 million years old.
They tend to be cherty, though chert can not be used as a way to classify iron formations because it is a common component in many types of rocks.
Iron formations are often associates with dolomite, quartz-rich sandstone, and black shale.
[1] In low-grade iron formations, there are different dominant minerals dependent on the different types of facies.
Most iron formations are deformed or metamorphosed simply due to their incredibly old age, but they still retain their unique distinctive chemical composition; even at high metamorphic grades.
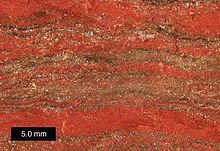
Banded iron formations (BIFs) were originally chemical muds and contain well developed thin lamination.
BIFs show regular alternating layers that are rich in iron and chert that range in thickness from a few millimeters to a few centimeters.
These formations can contain sedimentary structures like cross-bedding, graded bedding, load casts, ripple marks, mud cracks, and erosion channels.
In comparison to GIFs, BIFs contain a much larger spectrum of iron minerals, have more reduced facies, and are more abundant.
[1] BIFs are divided into type categories based on the characteristics related to the nature of their formation and unique physical and chemical properties.
[5] Algoma types are small lenticular iron deposits that are associated with volcanic rocks and turbidites.
[7] Superior types are large, thick, extensive iron deposits across stable shelves and in broad basins.
[7] Granular iron formations (GIFs) were originally well-sorted chemical sands.
They contain sand-sized clasts and a finer grained matrix, and generally belong to the oxide or silicate mineral facies.
[1] There are four facies types associated with iron-rich sedimentary rocks: oxide-, silicate-, carbonate-, and sulfide-facies.
[8] Commonly, the presence of iron is determined to be within a rock due to certain colorations from oxidation.
This form of iron is very stable structurally because its valence electron shell is half filled.
[9] Laterization is a soil forming process that occurs in warm and moist climates under broadleaf evergreen forests.
The process is caused by sulfate reduction which replaces carbonate skeletons (or shells) with pyrite (FeS2).
In freshwater environments, siderite will replace carbonate shells instead of pyrite due to the low amounts of sulfate.
In older samples, the ooids may be squished and have hooked tails on either end due to compaction.