Ferric
Relative to lower oxidation states, ferric is less common in organoiron chemistry, but the ferrocenium cation [Fe(C2H5)2]+ is well known.
Many organisms, from bacteria to humans, store iron as microscopic crystals (3 to 8 nm in diameter) of iron(III) oxide hydroxide, inside a shell of the protein ferritin, from which it can be recovered as needed.
Bacteria and grasses can thrive in such environments by secreting compounds called siderophores that form soluble complexes with iron(III), that can be reabsorbed into the cell.
Iron(III) nitrate dissolved in water to give [Fe(H2O)6]3+ ions.
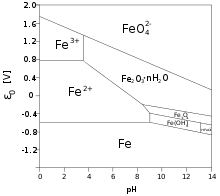
Eventually these solutions hydrolyze producing iron(III) hydroxide Fe(OH)3 that further converts to polymeric oxide-hydroxide via the process called olation.
Citrate also solubilizes ferric ion at neutral pH, although its complexes are less stable than those of EDTA.
Many chelating ligands - the siderophores - are produced naturally to dissolve iron(III) oxides.
Iron(III) is a d5 center, meaning that the metal has five "valence" electrons in the 3d orbital shell.
The number and type of ligands bound to iron(III) determine how these electrons arrange themselves.