Isobaric process
[1] Pressure-volume work by the closed system is defined as: where Δ means change over the whole process, whereas d denotes a differential.
According to the equipartition theorem,[2] the change in internal energy is related to the temperature of the system by where cV, m is molar heat capacity at a constant volume.
The formulas for specific heats would reduce in these special cases: Monatomic: Diatomic: An isobaric process is shown on a P–V diagram as a straight horizontal line, connecting the initial and final thermostatic states.
The motivation for the specific sign conventions of thermodynamics comes from early development of heat engines.
When designing a heat engine, the goal is to have the system produce and deliver work output.
It is called enthalpy, and is denoted as H. Therefore, an isobaric process can be more succinctly described as Enthalpy and isochoric specific heat capacity are very useful mathematical constructs, since when analyzing a process in an open system, the situation of zero work occurs when the fluid flows at constant pressure.
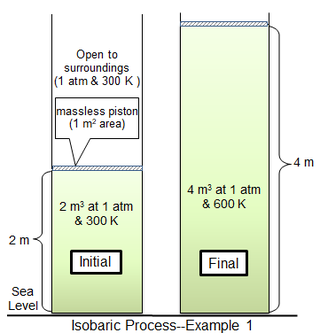
For the limiting case of a massless piston, the cylinder gas is also at 1 atm pressure, with an initial volume of 2 m3.
If the piston motion is sufficiently slow, the gas pressure at each instant will have practically the same value (psys = 1 atm) throughout.
For a thermally perfect diatomic gas, the molar specific heat capacity at constant pressure (cp) is 7/2R or 29.1006 J mol−1 deg−1.
The second process example is similar to the first, except that the massless piston is replaced by one having a mass of 10,332.2 kg, which doubles the pressure of the cylinder gas to 2 atm.
If the piston motion is sufficiently slow, the gas pressure at each instant will have practically the same value (psys = 2 atm) throughout.
Since enthalpy and internal energy are independent of pressure, As in the first example, about 28.6% of the supplied heat is converted to work.
In this context the ideal gas law is written where T is thermodynamic temperature and M is molar mass.