Equipartition theorem
For example, it predicts that every atom in a monatomic ideal gas has an average kinetic energy of 3/2kBT in thermal equilibrium, where kB is the Boltzmann constant and T is the (thermodynamic) temperature.
Although the equipartition theorem makes accurate predictions in certain conditions, it is inaccurate when quantum effects are significant, such as at low temperatures.
For example, the heat capacity of a solid decreases at low temperatures as various types of motion become frozen out, rather than remaining constant as predicted by equipartition.
Such decreases in heat capacity were among the first signs to physicists of the 19th century that classical physics was incorrect and that a new, more subtle, scientific model was required.
For example, it predicts that every atom of an inert noble gas, in thermal equilibrium at temperature T, has an average translational kinetic energy of 3/2kBT, where kB is the Boltzmann constant.
By exactly the same reasoning as in the translational case, equipartition implies that in thermal equilibrium the average rotational energy of each particle is 3/2kBT.
Similarly, the equipartition theorem allows the average (more precisely, the root mean square) angular speed of the molecules to be calculated.
[6] The tumbling of rigid molecules—that is, the random rotations of molecules in solution—plays a key role in the relaxations observed by nuclear magnetic resonance, particularly protein NMR and residual dipolar couplings.
Hence, a protein clump with a buoyant mass of 10 MDa (roughly the size of a virus) would produce a haze with an average height of about 2 cm at equilibrium.
[17] In 1876, Ludwig Boltzmann expanded on this principle by showing that the average energy was divided equally among all the independent components of motion in a system.
[18][19] Boltzmann applied the equipartition theorem to provide a theoretical explanation of the Dulong–Petit law for the specific heat capacities of solids.
Experiments confirmed the former prediction,[4] but found that molar heat capacities of diatomic gases were typically about 5 cal/(mol·K),[24] and fell to about 3 cal/(mol·K) at very low temperatures.
Boltzmann defended the derivation of his equipartition theorem as correct, but suggested that gases might not be in thermal equilibrium because of their interactions with the aether.
As well as providing the formula for the average kinetic energy per particle, the equipartition theorem can be used to derive the ideal gas law from classical mechanics.
Although equipartition provides a simple derivation of the ideal-gas law and the internal energy, the same results can be obtained by an alternative method using the partition function.
[36] A diatomic gas can be modelled as two masses, m1 and m2, joined by a spring of stiffness a, which is called the rigid rotor-harmonic oscillator approximation.
However, relativistic effects become dominant in some systems, such as white dwarfs and neutron stars,[10] and the ideal gas equations must be modified.
In these cases, the law of equipartition predicts that Thus, the average potential energy equals kBT/s, not kBT/2 as for the quadratic harmonic oscillator (where s = 2).
[40] As examples, the virial theorem may be used to estimate stellar temperatures or the Chandrasekhar limit on the mass of white dwarf stars.
However, the Sun is much more complex than assumed by this model—both its temperature and density vary strongly with radius—and such excellent agreement (≈7% relative error) is partly fortuitous.
[46] The original formulation of the equipartition theorem states that, in any physical system in thermal equilibrium, every particle has exactly the same average translational kinetic energy, 3/2kBT.
The law of equipartition holds only for ergodic systems in thermal equilibrium, which implies that all states with the same energy must be equally likely to be populated.
[50] A commonly cited counter-example where energy is not shared among its various forms and where equipartition does not hold in the microcanonical ensemble is a system of coupled harmonic oscillators.
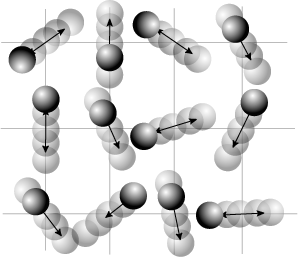
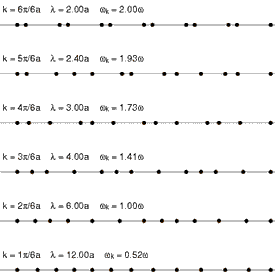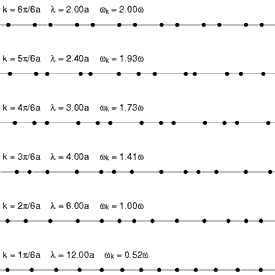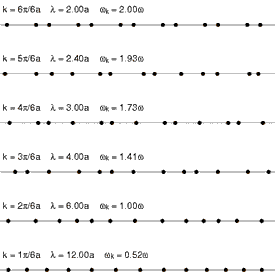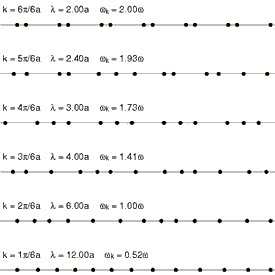
This puzzling result was eventually explained by Kruskal and Zabusky in 1965 in a paper which, by connecting the simulated system to the Korteweg–de Vries equation led to the development of soliton mathematics.
[12] To illustrate the breakdown of equipartition, consider the average energy in a single (quantum) harmonic oscillator, which was discussed above for the classical case.
As another example, the internal excited electronic states of a hydrogen atom do not contribute to its specific heat as a gas at room temperature, since the thermal energy kBT (roughly 0.025 eV) is much smaller than the spacing between the lowest and next higher electronic energy levels (roughly 10 eV).
[52] The paradox arises because there are an infinite number of independent modes of the electromagnetic field in a closed container, each of which may be treated as a harmonic oscillator.
For example, the valence electrons in a metal can have a mean kinetic energy of a few electronvolts, which would normally correspond to a temperature of tens of thousands of kelvins.
Such a state, in which the density is high enough that the Pauli exclusion principle invalidates the classical approach, is called a degenerate fermion gas.
[citation needed] At low temperatures, a fermionic analogue of the Bose–Einstein condensate (in which a large number of identical particles occupy the lowest-energy state) can form; such superfluid electrons are responsible[dubious – discuss] for superconductivity.