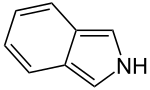
Isoindole
The parent isoindole is a rarely encountered in the technical literature, but substituted derivatives are useful commercially and occur naturally.
[3][4] The parent isoindole was prepared by flash vacuum pyrolysis of an N-substituted isoindoline.
Unlike indole, isoindoles exhibit noticeable alternation in the C-C bond lengths, which is consistent with their description as pyrrole derivatives fused to a butadiene.
[6] The degree to which the 2H predominates depends on the solvent, and can vary with the substituent in substituted isoindoles.
The commercially important phthalimide is an isoindole-1,3-dione with two carbonyl groups attached to the heterocyclic ring.