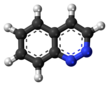
Cinnoline
It is isomeric with other naphthyridines including quinoxaline, phthalazine and quinazoline.
It has a taste resembling that of chloral hydrate and leaves a sharp irritation for some time.
The compound was first obtained in impure form by cyclization of the alkyne o-C6H4(N2Cl)C≡CCO2H in water to give 4-hydroxycinnoline-3-carboxylic acid.
This material could be decarboxylated and the hydroxyl group reductively removed to give the parent heterocycle.
It can be prepared by dehydrogenation of dihydrocinnoline with freshly precipitated mercuric oxide.