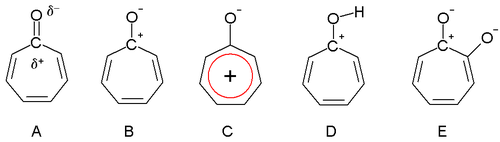
Tropone
The related compound tropolone (2-hydroxy-2,4,6-cycloheptatrien-1-one) has an additional alcohol (or an enol including the double bond) group next to the ketone.
In an extreme case, the carbon atom has a full positive charge (B) forming a tropylium ion ring which is an aromatic 6 electron system (C).
Two selected methods for the synthesis of tropone are by selenium dioxide oxidation of cycloheptatriene[4] and indirectly from tropinone by a Hofmann elimination and a bromination.
[2] Tropone undergoes ring contraction to benzoic acid with potassium hydroxide at elevated temperature.
[6] Other tropone derivatives include puberulonic and puberulic acids, roseobacticides, pernambucone, crototropone, orobanone.